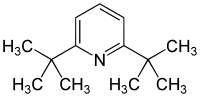
2,6-Di-tert-butylpyridine
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2,6-Di-tert-butylpyridine | |
| Other names
Dibutylpyridine | |
| Identifiers | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 61785 |
| ECHA InfoCard | 100.008.690 |
| PubChem | 68510 |
| |
| |
| Properties | |
| C13H21N | |
| Molar mass | 191.3125 |
| Appearance | colourless liquid |
| Density | 0.885 g/cm3 |
| Hazards | |
| Flash point | 72.2 °C (162.0 °F; 345.3 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
2,6-Di-tert-butylpyridine is an organic compound with the formula (Me3C)2C5H3N. This colourless, oily liquid is derived from pyridine by replacement of the two H atoms with tert-butyl groups. It is a hindered base.[1] For example, it can be protonated, but it does not form an adduct with boron trifluoride.
Preparation
2,6-Di-tert-butylpyridine is prepared by the reaction of tert-butyllithium with pyridine.[2] The synthesis is reminiscent of the Chichibabin reaction.
Some related bulky pyridine compounds have been described, including 2,4,6-tri-t-butylpyridine.[3] and 2,6-di-tert-butyl-4-methylpyridine.[4]
References
- ↑ Rafael R. Kostikov, Sánchez-Sancho Francisco, María Garranzo and M. Carmen Murcia "2,6-Di-t-butylpyridine" Encyclopedia of Reagents for Organic Synthesis 2010. doi:10.1002/047084289X.rd068.pub2
- ↑ Edward Deutsch, Nai Kong V. Cheung "Noncoordinating buffers. I. Synthesis and characterization of water soluble derivatives of 2,6-di-tert-butylpyridine" J. Org. Chem 1973, vol 38, pp 1123–1126. doi:10.1021/jo00946a013
- ↑ Francis V. Scalzi, Norman F. Golob "Alkylation of pyridine with tert-butyllithium. Convenient syntheses of 2,6-di-tert-butylpyridine and 2,4,6-tri-tert-butylpyridine" J. Org. Chem 1971, vol 36, pp 2541–2542 doi:10.1021/jo00816a036. Hongmei Li "2,4,6-Tri-tert-butylpyridine" Encyclopedia of Reagents for Organic Synthesis 2004. doi:10.1002/047084289X.rn00512
- ↑ Alexandru T. Balaban "2,6-Di-tert-butyl-4-methylpyridine (DTBMP)" Encyclopedia of Reagents for Organic Synthesis 2004. doi:10.1002/047084289X.rn00509
This article is issued from Wikipedia - version of the 9/16/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.