Lysergic acid diethylamide
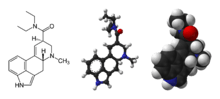 2D structural formula and 3D models of LSD | |
| Clinical data | |
|---|---|
| Pronunciation | /daɪ eθəl ˈæmaɪd/, /æmɪd/, or /eɪmaɪd/)[1][2][3] |
| Pregnancy category |
|
| Dependence liability | Low[4] |
| Addiction liability | None[5] |
| Routes of administration | Oral, sublingual, intravenous, ocular, intramuscular |
| ATC code | none |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Biological half-life | 3–5 hours[6][7] |
| Excretion | Renal |
| Identifiers | |
| |
| Synonyms | Acid, LSD, lysergide |
| CAS Number |
50-37-3 |
| PubChem (CID) | 5761 |
| IUPHAR/BPS | 17 |
| DrugBank |
DB04829 |
| ChemSpider |
5558 |
| UNII |
8NA5SWF92O |
| ChEBI |
CHEBI:6605 |
| ChEMBL |
CHEMBL263881 |
| Chemical and physical data | |
| Formula | C20H25N3O |
| Molar mass | 323.44 g·mol−1 |
| 3D model (Jmol) | Interactive image |
| Melting point | 80 to 85 °C (176 to 185 °F) |
| |
| |
| (verify) | |
Lysergic acid diethylamide (LSD), also known as acid, is a psychedelic drug known for its psychological effects. This may include altered awareness of the surroundings, perceptions, and feelings as well as sensations and images that seem real though they are not.[8] It is used mainly as a recreational drug and for spiritual reasons. LSD is typically either swallowed or held under the tongue.[8] It is often sold on blotter paper, a sugar cube, or gelatin. It can also be injected.
LSD is not addictive.[8][9] However, adverse psychiatric reactions such as anxiety, paranoia, and delusions are possible.[10] LSD is in the ergoline family. LSD is sensitive to oxygen, ultraviolet light, and chlorine,[11] though it may last for years if it is stored away from light and moisture at low temperature. In pure form it is odorless and clear or white in color.[8] As little as 20–30 micrograms can produce an effect.[12]
LSD was first made by Albert Hofmann in Switzerland in 1938 from ergotamine, a chemical from the fungus, ergot. The laboratory name for the compound was the acronym for the German "Lyserg-säure-diäthylamid", followed by a sequential number: LSD-25.[11][13] Hofmann discovered its psychedelic properties in 1943.[14] LSD was introduced as a commercial medication under the trade-name Delysid for various psychiatric uses in 1947.[15] In the 1950s, officials at the U.S. Central Intelligence Agency (CIA) thought the drug might be useful for mind control and chemical warfare and tested the drug on young servicemen and students, and others without their knowledge. The subsequent recreational use by youth culture in the Western world as part of 1960s counterculture resulted in its prohibition.[16]
Uses
Medical
LSD currently has no approved uses in medicine.[17][18]
Recreational

LSD is commonly used as a recreational drug.[19]
Spiritual
LSD is considered an entheogen because it can catalyze intense spiritual experiences, during which users may feel they have come into contact with a greater spiritual or cosmic order. Users sometimes report out of body experiences. In 1966, Timothy Leary established the League for Spiritual Discovery with LSD as its sacrament.[20][21] Stanislav Grof has written that religious and mystical experiences observed during LSD sessions appear to be phenomenologically indistinguishable from similar descriptions in the sacred scriptures of the great religions of the world and the texts of ancient civilizations.[22]
Effects
Physical
LSD can cause pupil dilation, reduced appetite, and wakefulness. Other physical reactions to LSD are highly variable and nonspecific, some of which may be secondary to the psychological effects of LSD. Among the reported symptoms are numbness, weakness, nausea, hypothermia or hyperthermia, elevated blood sugar, goose bumps, heart rate increase, jaw clenching, perspiration, saliva production, mucus production, hyperreflexia, and tremors.
Psychological
The most common immediate psychological effects of LSD are visual hallucinations and illusions (colloquially known as "trips"), which can vary greatly depending on how much is used and how the brain responds. Trips usually start within 20–30 minutes of taking LSD by mouth (less if snorted or taken intravenously), peak three to four hours after ingestion, and last up to 12 hours. Negative experiences, referred to as "bad trips", produce intense negative emotions, such as irrational fears and anxiety, panic attacks, paranoia, rapid mood swings, intrusive thoughts of hopelessness, wanting to harm others, and suicidal ideation. It is impossible to predict when a bad trip will occur.[25][26] Good trips are simulating and pleasurable, and typically involve feeling as if one is floating, disconnected from reality, feelings of joy or euphoria (sometimes called a "rush"), decreased inhibitions, and the belief that one has extreme mental clarity or superpowers.[25]
Sensory
Some sensory effects may include an experience of radiant colors, objects and surfaces appearing to ripple or "breathe", colored patterns behind the closed eyelids (eidetic imagery), an altered sense of time (time seems to be stretching, repeating itself, changing speed or stopping), crawling geometric patterns overlaying walls and other objects, and morphing objects.[27] Some users, including Albert Hofmann, report a strong metallic taste for the duration of the effects.[28]
LSD causes an animated sensory experience of senses, emotions, memories, time, and awareness for 6 to 14 hours, depending on dosage and tolerance. Generally beginning within 30 to 90 minutes after ingestion, the user may experience anything from subtle changes in perception to overwhelming cognitive shifts. Changes in auditory and visual perception are typical.[27][29] Visual effects include the illusion of movement of static surfaces ("walls breathing"), after image-like trails of moving objects ("tracers"), the appearance of moving colored geometric patterns (especially with closed eyes), an intensification of colors and brightness ("sparkling"), new textures on objects, blurred vision, and shape suggestibility. Users commonly report that the inanimate world appears to animate in an inexplicable way; for instance, objects that are static in three dimensions can seem to be moving relative to one or more additional spatial dimensions.[30] Many of the basic visual effects resemble the phosphenes seen after applying pressure to the eye and have also been studied under the name "form constants". The auditory effects of LSD may include echo-like distortions of sounds, changes in ability to discern concurrent auditory stimuli, and a general intensification of the experience of music. Higher doses often cause intense and fundamental distortions of sensory perception such as synaesthesia, the experience of additional spatial or temporal dimensions, and temporary dissociation.
Adverse effects
Mental disorders
LSD may trigger panic attacks or feelings of extreme anxiety, known familiarly as a "bad trip." Review studies suggest that LSD likely plays a role in precipitating the onset of acute psychosis in previously healthy individuals with an increased likelihood in individuals who have a family history of schizophrenia.[10][32] There is evidence that people with severe mental illnesses like schizophrenia have a higher likelihood of experiencing adverse effects from taking LSD.[32]
Suggestibility
While publicly available documents indicate that the CIA and Department of Defense have discontinued research into the use of LSD as a means of mind control,[33] research from the 1960s suggests that both mentally ill and healthy people are more suggestible while under its influence.[34][35]
Flashbacks
Some individuals may experience "flashbacks" and a syndrome of long-term and occasionally distressing perceptual changes.[36][37]
"Flashbacks" are a reported psychological phenomenon in which an individual experiences an episode of some of LSD's subjective effects after the drug has worn off, "persisting for months or years after hallucinogen use".[38] Several studies have tried to determine the likelihood that a user of LSD, not suffering from known psychiatric conditions, will experience flashbacks. The larger studies include Blumenfeld's in 1971[38][39][40][41][42] and Naditch and Fenwick's in 1977,[43][44][45][46][47] which arrived at figures of 20%[39] and 28%,[43] respectively.[36][48][49]
Hallucinogen persisting perception disorder (HPPD) describes a post-LSD exposure syndrome in which LSD-like visual changes are not temporary and brief, as they are in flashbacks, but instead are persistent, and cause clinically significant impairment or distress. The syndrome is a DSM-IV diagnosis. Several scientific journal articles have described the disorder.[50] HPPD differs from flashbacks in that it is persistent and apparently entirely visual (although mood and anxiety disorders are sometimes diagnosed in the same individuals). A recent review suggests that HPPD (as defined in the DSM-IV) is uncommon and affects a distinctly vulnerable subpopulation of users.[51][52]
Pregnancy
Early pharmacological testing by Sandoz in laboratory animals showed that LSD can stimulate uterine contractions, with efficacy comparable to ergobasine, the active uterotonic component of the ergot fungus.
The mutagenic potential of LSD is unclear. Overall, the evidence seems to point to limited or no effect at commonly used doses.[53]
Tolerance
Tolerance to LSD builds up over consistent use[54] and cross-tolerance has been demonstrated between LSD, mescaline[55] and psilocybin.[56] This tolerance is probably caused by downregulation of 5-HT2A receptors in the brain and diminishes a few days after cessation of use.
LSD is not addictive.[5][9][57] Experimental evidence has demonstrated that LSD use does not yield positive reinforcement in either human or animal subjects.[5][58][59]
Overdose
Reassurance in a calm, safe environment is beneficial. Agitation can be safely addressed with benzodiazepines such as lorazepam or diazepam. Neuroleptics such as haloperidol are recommended against because they may have adverse effects. LSD is rapidly absorbed, so activated charcoal and emptying of the stomach will be of little benefit, unless done within 30–60 minutes of ingesting an overdose of LSD. Sedation or physical restraint is rarely required, and excessive restraint may cause complications such as hyperthermia (over-heating) or rhabdomyolysis.[60]
Massive doses require supportive care, which may include endotracheal intubation or respiratory support.[60] Overdose has been recorded at 1,000 to 7,000μg per 100ml & 2.1 to 26 ng per ml in serum concentrations of the tartrate salt form. It is recommended that high blood pressure, tachycardia (rapid heart-beat), and hyperthermia, if present, are treated symptomatically, and that low blood pressure is treated initially with fluids and then with pressors if necessary. Intravenous administration of anticoagulants, vasodilators, and sympatholytics may be useful with massive doses.[60][61]
Pharmacology
Pharmacodynamics
LSD affects a large number of the G protein-coupled receptors, including all dopamine receptor subtypes, and all adrenoreceptor subtypes, as well as many others. Most serotonergic psychedelics are not significantly dopaminergic, and LSD is therefore rather unusual in this regard. LSD's agonism of D2 receptors contributes to its psychoactive effects.[62][63] LSD binds to most serotonin receptor subtypes except for 5-HT3 and 5-HT4. However, most of these receptors are affected at too low affinity to be sufficiently activated by the brain concentration of approximately 10–20 nM.[57] In humans, recreational doses of LSD can affect 5-HT1A (Ki=1.1nM), 5-HT2A (Ki=2.9nM), 5-HT2B (Ki=4.9nM), 5-HT2C (Ki=23nM), 5-HT5A (Ki=9nM [in cloned rat tissues]), and 5-HT6 receptors (Ki=2.3nM).[6][64] 5-HT5B receptors, which are not present in humans, also have a high affinity for LSD.[65] The psychedelic effects of LSD are attributed to cross-activation of 5-HT2A receptor heteromers.[66] Many but not all 5-HT2A agonists are psychedelics and 5-HT2A antagonists block the psychedelic activity of LSD. LSD exhibits functional selectivity at the 5-HT2A and 5HT2C receptors in that it activates the signal transduction enzyme phospholipase A2 instead of activating the enzyme phospholipase C as the endogenous ligand serotonin does.[67] Exactly how LSD produces its effects is unknown, but it is thought that it works by increasing glutamate release in the cerebral cortex[57] and therefore excitation in this area, specifically in layers IV and V.[68] LSD, like many other drugs, has been shown to activate DARPP-32-related pathways.[69]
LSD enhances dopamine D2R protomer recognition and signaling of D2–5-HT2A receptor complexes. This mechanism may contribute to the psychotic actions of LSD.[70]
Pharmacokinetics
LSD's effects normally last from 6–12 hours depending on dosage, tolerance, body weight and age.[11] The Sandoz prospectus for "Delysid" warned: "intermittent disturbances of affect may occasionally persist for several days."[13] Contrary to early reports and common belief, LSD effects do not last longer than the amount of time significant levels of the drug are present in the blood. Aghajanian and Bing (1964) found LSD had an elimination half-life of only 175 minutes.[6] However, using more accurate techniques, Papac and Foltz (1990) reported that 1 µg/kg oral LSD given to a single male volunteer had an apparent plasma half-life of 5.1 hours, with a peak plasma concentration of 5 ng/mL at 3 hours post-dose.[7]
Chemistry

LSD is a chiral compound with two stereocenters at the carbon atoms C-5 and C-8, so that theoretically four different optical isomers of LSD could exist. LSD, also called (+)-D-LSD, has the absolute configuration (5R,8R). The C-5 isomers of lysergamides do not exist in nature and are not formed during the synthesis from D-lysergic acid. Retrosynthetically, the C-5 stereocenter could be analysed as having the same configuration of the alpha carbon of the naturally occurring amino acid L-tryptophan, the precursor to all biosynthetic ergoline compounds.
However, LSD and iso-LSD, the two C-8 isomers, rapidly interconvert in the presence of bases, as the alpha proton is acidic and can be deprotonated and reprotonated. Non-psychoactive iso-LSD which has formed during the synthesis can be separated by chromatography and can be isomerized to LSD.
Pure salts of LSD are triboluminescent, emitting small flashes of white light when shaken in the dark.[11] LSD is strongly fluorescent and will glow bluish-white under UV light.
Synthesis
LSD is an ergoline derivative. It is commonly synthesized by reacting diethylamine with an activated form of lysergic acid. Activating reagents include phosphoryl chloride[71] and peptide coupling reagents.[72] Lysergic acid is made by alkaline hydrolysis of lysergamides like ergotamine, a substance usually derived from the ergot fungus on agar plate; or, theoretically possible, but impractical and uncommon, from ergine (lysergic acid amide, LSA) extracted from morning glory seeds.[73] Lysergic acid can also be produced synthetically, eliminating the need for ergotamines.[74][75]
Dosage

A single dose of LSD may be between 40 and 500 micrograms—an amount roughly equal to one-tenth the mass of a grain of sand. Threshold effects can be felt with as little as 25 micrograms of LSD.[12][76] Dosages of LSD are measured in micrograms (µg), or millionths of a gram. By comparison, dosages of most drugs, both recreational and medicinal, are measured in milligrams (mg), or thousandths of a gram. For example, an active dose of mescaline, roughly 0.2 to 0.5 g, has effects comparable to 100 µg or less of LSD.[13]
In the mid-1960s, the most important black market LSD manufacturer (Owsley Stanley) distributed acid at a standard concentration of 270 µg,[77] while street samples of the 1970s contained 30 to 300 µg. By the 1980s, the amount had reduced to between 100 and 125 µg, dropping more in the 1990s to the 20–80 µg range,[78] and even more in the 2000s (decade).[77][79]
Reactivity and degradation
"LSD," writes the chemist Alexander Shulgin, "is an unusually fragile molecule… As a salt, in water, cold, and free from air and light exposure, it is stable indefinitely."[11]
LSD has two labile protons at the tertiary stereogenic C5 and C8 positions, rendering these centres prone to epimerisation. The C8 proton is more labile due to the electron-withdrawing carboxamide attachment, but removal of the chiral proton at the C5 position (which was once also an alpha proton of the parent molecule tryptophan) is assisted by the inductively withdrawing nitrogen and pi electron delocalisation with the indole ring.
LSD also has enamine-type reactivity because of the electron-donating effects of the indole ring. Because of this, chlorine destroys LSD molecules on contact; even though chlorinated tap water contains only a slight amount of chlorine, the small quantity of compound typical to an LSD solution will likely be eliminated when dissolved in tap water.[11] The double bond between the 8-position and the aromatic ring, being conjugated with the indole ring, is susceptible to nucleophilic attacks by water or alcohol, especially in the presence of light. LSD often converts to "lumi-LSD", which is inactive in human beings.[11]
A controlled study was undertaken to determine the stability of LSD in pooled urine samples.[80] The concentrations of LSD in urine samples were followed over time at various temperatures, in different types of storage containers, at various exposures to different wavelengths of light, and at varying pH values. These studies demonstrated no significant loss in LSD concentration at 25 °C for up to four weeks. After four weeks of incubation, a 30% loss in LSD concentration at 37 °C and up to a 40% at 45 °C were observed. Urine fortified with LSD and stored in amber glass or nontransparent polyethylene containers showed no change in concentration under any light conditions. Stability of LSD in transparent containers under light was dependent on the distance between the light source and the samples, the wavelength of light, exposure time, and the intensity of light. After prolonged exposure to heat in alkaline pH conditions, 10 to 15% of the parent LSD epimerized to iso-LSD. Under acidic conditions, less than 5% of the LSD was converted to iso-LSD. It was also demonstrated that trace amounts of metal ions in buffer or urine could catalyze the decomposition of LSD and that this process can be avoided by the addition of EDTA.
Detection in body fluids
LSD may be quantified in urine as part of a drug abuse testing program, in plasma or serum to confirm a diagnosis of poisoning in hospitalized victims or in whole blood to assist in a forensic investigation of a traffic or other criminal violation or a case of sudden death. Both the parent drug and its major metabolite are unstable in biofluids when exposed to light, heat or alkaline conditions and therefore specimens are protected from light, stored at the lowest possible temperature and analyzed quickly to minimize losses.[81]
The apparent plasma half life of LSD is considered to be around 5.1 hours with peak plasma concentrations occurring 3 hours after administration.[82]
History
"... affected by a remarkable restlessness, combined with a slight dizziness. At home I lay down and sank into a not unpleasant intoxicated-like condition, characterized by an extremely stimulated imagination. In a dreamlike state, with eyes closed (I found the daylight to be unpleasantly glaring), I perceived an uninterrupted stream of fantastic pictures, extraordinary shapes with intense, kaleidoscopic play of colors. After some two hours this condition faded away."
Albert Hofmann, on his first experience with LSD[83]
LSD was first synthesized on November 16, 1938[84] by Swiss chemist Albert Hofmann at the Sandoz Laboratories in Basel, Switzerland as part of a large research program searching for medically useful ergot alkaloid derivatives. LSD's psychedelic properties were discovered 5 years later when Hofmann himself accidentally ingested an unknown quantity of the chemical.[85] The first intentional ingestion of LSD occurred on April 19, 1943,[86] when Hofmann ingested 250 µg of LSD. He said this would be a threshold dose based on the dosages of other ergot alkaloids. Hofmann found the effects to be much stronger than he anticipated.[87] Sandoz Laboratories introduced LSD as a psychiatric drug in 1947.[88]
Beginning in the 1950s, the US Central Intelligence Agency began a research program code named Project MKULTRA. Experiments included administering LSD to CIA employees, military personnel, doctors, other government agents, prostitutes, mentally ill patients, and members of the general public in order to study their reactions, usually without the subjects' knowledge. The project was revealed in the US congressional Rockefeller Commission report in 1975.
In 1963, the Sandoz patents expired on LSD.[78] Several figures, including Aldous Huxley, Timothy Leary, and Al Hubbard, began to advocate the consumption of LSD. LSD became central to the counterculture of the 1960s.[89] In the early 1960s the use of LSD and other hallucinogens was advocated by new proponents of consciousness expansion such as Leary, Huxley, Alan Watts and Arthur Koestler,[90][91] and according to L. R. Veysey they profoundly influenced the thinking of the new generation of youth.[92]
The Grateful Dead were inextricably linked to LSD in the United States, and Grateful Dead concerts provided the primary distribution network for LSD through the mid-1990s.[93]
On October 24, 1968, possession of LSD was made illegal in the United States.[94] The last FDA approved study of LSD in patients ended in 1980, while a study in healthy volunteers was made in the late 1980s. Legally approved and regulated psychiatric use of LSD continued in Switzerland until 1993.[95]
Society and culture
Counterculture, music and art

By the mid-1960s, the youth countercultures in California, particularly in San Francisco, had adopted the use of hallucinogenic drugs, with the first major underground LSD factory established by Owsley Stanley.[96] From 1964, the Merry Pranksters, a loose group that developed around novelist Ken Kesey, sponsored the Acid Tests, a series of events primarily staged in or near San Francisco, involving the taking of LSD (supplied by Stanley), accompanied by light shows, film projection and discordant, improvised music known as the psychedelic symphony.[97][98] The Pranksters helped popularize LSD use, through their road trips across America in a psychedelically-decorated converted school bus, which involved distributing the drug and meeting with major figures of the beat movement, and through publications about their activities such as Tom Wolfe's The Electric Kool-Aid Acid Test (1968).[99] In both music and art, the influence on LSD was soon being more widely seen and heard thanks to the bands that participated in the Acid Tests and related events, including the Grateful Dead, Jefferson Airplane and Big Brother and the Holding Company, and through the inventive poster and album art of San Francisco-based artists like Rick Griffin, Victor Moscoso, Bonnie MacLean, Stanley Mouse & Alton Kelley, and Wes Wilson, meant to evoke the visual experience of an LSD trip.
In San Francisco's Haight-Ashbury neighborhood, brothers Ron and Jay Thelin opened the Psychedelic Shop in January 1966. The Thelins' store is regarded as the first ever head shop. The Thelins opened the store to promote safe use of LSD, which was then still legal in California. The Psychedelic Shop helped to further popularize LSD in the Haight and to make the neighborhood the unofficial capital of the hippie counterculture in the United States. Ron Thelin was also involved in organizing the Love Pageant rally, a protest held in Golden Gate park to protest California's newly adopted ban on LSD in October 1966. At the rally, hundreds of attendees took acid in unison. Although the Psychedelic Shop closed after barely a year-and-a-half in business, its role in popularizing LSD was considerable.[100]
A similar and connected nexus of LSD use in the creative arts developed around the same time in London. A key figure in this phenomenon in the UK was British academic Michael Hollingshead, who first tried LSD in America 1961 while he was the Executive Secretary for the Institute of British-American Cultural Exchange. After being given a large quantity of pure Sandoz LSD (which was still legal at the time) and experiencing his first "trip", Hollingshead contacted Aldous Huxley, who suggested that he get in touch with Harvard academic Timothy Leary, and over the next few years, in concert with Leary and Richard Alpert, Hollingshead played a major role in their famous LSD research at Millbrook before moving to New York City, where he conducted his own LSD experiments. In 1965 Hollingshead returned to the UK and founded the World Psychedelic Center in Chelsea, London. Among the many famous people in the UK that Hollingshead is reputed to have introduced to LSD are artist and Hipgnosis founder Storm Thorgerson, and musicians Donovan, Keith Richards, Paul McCartney, John Lennon, and George Harrison. Although establishment concern about the new drug led to it being declared an illegal drug by the Home Secretary in 1966, LSD was soon being used widely in the upper echelons of the British art and music scene, including members of the Beatles, the Rolling Stones, the Moody Blues, the Small Faces, Pink Floyd, Jimi Hendrix and others, and the products of these experiences were soon being both heard and seen by the public with singles like The Small Faces' "Itchycoo Park" and LPs like the Beatles' Sgt Pepper's Lonely Hearts Club Band and Cream's Disraeli Gears, which featured music that showed the obvious influence of the musicians' recent psychedelic excursions, and which were packaged in elaborately-designed album covers that featured vividly-coloured psychedelic artwork by artists like Peter Blake, Martin Sharp, Hapshash and the Coloured Coat (Nigel Waymouth and Michael English) and art/music collective "The Fool".
In the 1960s, musicians from psychedelic music and psychedelic rock bands began to refer (at first indirectly, and later explicitly) to the drug and attempted to recreate or reflect the experience of taking LSD in their music. A number of features are often included in psychedelic music. Exotic instrumentation, with a particular fondness for the sitar and tabla are common.[101] Electric guitars are used to create feedback, and are played through wah wah and fuzzbox effect pedals.[102] Elaborate studio effects are often used, such as backwards tapes, panning, phasing, long delay loops, and extreme reverb.[103] In the 1960s there was a use of primitive electronic instruments such as early synthesizers and the theremin.[104][105] Later forms of electronic psychedelia also employed repetitive computer-generated beats.[106] Songs referring to LSD include the Beatles' Lucy in the Sky with Diamonds. Psychedelic experiences were also reflected in psychedelic art, literature and film.[107]
LSD had a strong influence on the Grateful Dead and the culture of Deadheads, as well as the impact for artist Keith Haring, early techno music, and the jam band Phish.[93]
Legal status
The United Nations Convention on Psychotropic Substances (adopted in 1971) requires the signing parties to prohibit LSD. Hence, it is illegal in all countries that were parties to the convention, including the United States, Australia, New Zealand, and most of Europe. However, enforcement of those laws varies from country to country. Medical and scientific research with LSD in humans is permitted under the 1971 UN Convention.[108]
Australia
LSD is a Schedule 9 prohibited substance in Australia under the Poisons Standard (July 2016).[109] A Schedule 9 substance is a substance which may be abused or misused, the manufacture, possession, sale or use of which should be prohibited by law except when required for medical or scientific research, or for analytical, teaching or training purposes with approval of Commonwealth and/or State or Territory Health Authorities.[109]
In Western Australia section 9 of the Misuse of Drugs Act 1981 provides for summary trial before a magistrate for possession of less than 0.004g; section 11 provides rebuttable presumptions of intent to sell or supply if the quantity is 0.002g or more, or of possession for the purpose of trafficking if 0.01g.[110]
Canada
In Canada, LSD is a controlled substance under Schedule III of the Controlled Drugs and Substances Act.[111] Every person who seeks to obtain the substance, without disclosing authorization to obtain such substances 30 days before obtaining another prescription from a practitioner, is guilty of an indictable offense and liable to imprisonment for a term not exceeding 3 years. Possession for purpose of trafficking is an indictable offense punishable by imprisonment for 10 years.
United Kingdom
In the United Kingdom, LSD is a Schedule 1 Class 'A' drug. This means it has no recognized legitimate uses and possession of the drug without a license is punishable with 7 years' imprisonment and/or an unlimited fine, and trafficking is punishable with life imprisonment and an unlimited fine (see main article on drug punishments Misuse of Drugs Act 1971).
In 2000, after consultation with members of the Royal College of Psychiatrists' Faculty of Substance Misuse, the UK Police Foundation issued the Runciman Report which recommended "the transfer of LSD from Class A to Class B".[112]
In November 2009, the UK Transform Drug Policy Foundation released in the House of Commons a guidebook to the legal regulation of drugs, After the War on Drugs: Blueprint for Regulation, which details options for regulated distribution and sale of LSD and other psychedelics.[113]
United States
LSD is Schedule I in the United States, according to the Controlled Substances Act of 1970.[114] This means LSD is illegal to manufacture, buy, possess, process, or distribute without a license from the Drug Enforcement Administration (DEA). By classifying LSD as a Schedule I substance, the DEA holds that LSD meets the following three criteria: it is deemed to have a high potential for abuse; it has no legitimate medical use in treatment; and there is a lack of accepted safety for its use under medical supervision. There are no documented deaths from chemical toxicity; most LSD deaths are a result of behavioral toxicity.[115]
There can also be substantial discrepancies between the amount of chemical LSD that one possesses and the amount of possession with which one can be charged in the U.S. This is because LSD is almost always present in a medium (e.g. blotter or neutral liquid), and the amount that can be considered with respect to sentencing is the total mass of the drug and its medium. This discrepancy was the subject of 1995 United States Supreme Court case, Neal v. U.S.[116]
Lysergic acid and lysergic acid amide, LSD precursors, are both classified in Schedule III of the Controlled Substances Act. Ergotamine tartrate, a precursor to lysergic acid, is regulated under the Chemical Diversion and Trafficking Act.
Mexico
In April 2009, the Mexican Congress approved changes in the General Health Law that decriminalized the possession of illegal drugs for immediate consumption and personal use, allowing a person to possess a moderate amount of LSD. The only restriction is that people in possession of drugs should not be within a 300-meter radius of schools, police departments, or correctional facilities. Marijuana, along with cocaine, opium, heroin, and other drugs were also decriminalized, it will not be considered as a crime as long as the dose does not exceed the limit established in the General Health Law.[117] Many question this, as cocaine is as much synthesised as heroin, both are produced as extracts from plants. The law establishes very low amount thresholds and strictly defines personal dosage. For those arrested with more than the threshold allowed by the law this can result in heavy prison sentences, as they will be assumed to be small traffickers even if there are no other indications that the amount was meant for selling.[118]
Czech Republic
In the Czech Republic, until 31 December 1998 only drug possession "for other person" (i.e. intent to sell) was criminal (apart from production, importation, exportation, offering or mediation, which was and remains criminal) while possession for personal use remained legal.[119]
On 1 January 1999, an amendment of the Criminal Code, which was necessitated in order to align the Czech drug rules with the Single Convention on Narcotic Drugs, became effective, criminalizing possession of "amount larger than small" also for personal use (Art. 187a of the Criminal Code) while possession of small amounts for personal use became a misdemeanor.[119]
The judicial practice came to the conclusion that the "amount larger than small" must be five to ten times larger (depending on drug) than a usual single dose of an average consumer.[120]
Under the Regulation No. 467/2009 Coll, possession of more than 5 doses of LSD was to be considered smaller than large for the purposes of the Criminal Code and was to be treated as a misdemeanor subject to a fine equal to a parking ticket.[121]
Ecuador
According to the 2008 Constitution of Ecuador, in its Article 364, the Ecuadorian state does not see drug consumption as a crime but only as a health concern.[122] Since June 2013 the State drugs regulatory office CONSEP has published a table which establishes maximum quantities carried by persons so as to be considered in legal possession and that person as not a seller of drugs.[122][123][124] The "CONSEP established, at their latest general meeting, that the 0.020 milligrams of LSD shal be considered the maximum consumer amount.[125]
Economics
Production

An active dose of LSD is very minute, allowing a large number of doses to be synthesized from a comparatively small amount of raw material. Twenty five kilograms of precursor ergotamine tartrate can produce 5–6 kg of pure crystalline LSD; this corresponds to 100 million doses. Because the masses involved are so small, concealing and transporting illicit LSD is much easier than smuggling cocaine, cannabis, or other illegal drugs.[126]
Manufacturing LSD requires laboratory equipment and experience in the field of organic chemistry. It takes two to three days to produce 30 to 100 grams of pure compound. It is believed that LSD is not usually produced in large quantities, but rather in a series of small batches. This technique minimizes the loss of precursor chemicals in case a step does not work as expected.[126]
Forms

LSD is produced in crystalline form and then mixed with excipients or redissolved for production in ingestible forms. Liquid solution is either distributed in small vials or, more commonly, sprayed onto or soaked into a distribution medium. Historically, LSD solutions were first sold on sugar cubes, but practical considerations forced a change to tablet form. Appearing in 1968 as an orange tablet measuring about 6 mm across, "Orange Sunshine" acid was the first largely available form of LSD after its possession was made illegal. Tim Scully, a prominent chemist, made some of these tablets, but said that most "Sunshine" in the USA came by way of Ronald Stark, who imported approximately thirty-five million doses from Europe.[127]
Over a period of time, tablet dimensions, weight, shape and concentration of LSD evolved from large (4.5–8.1 mm diameter), heavyweight (≥150 mg), round, high concentration (90–350 µg/tab) dosage units to small (2.0–3.5 mm diameter) lightweight (as low as 4.7 mg/tab), variously shaped, lower concentration (12–85 µg/tab, average range 30–40 µg/tab) dosage units. LSD tablet shapes have included cylinders, cones, stars, spacecraft, and heart shapes. The smallest tablets became known as "Microdots".[128]
After tablets came "computer acid" or "blotter paper LSD", typically made by dipping a preprinted sheet of blotting paper into an LSD/water/alcohol solution.[127][128] More than 200 types of LSD tablets have been encountered since 1969 and more than 350 blotter paper designs have been observed since 1975.[128] About the same time as blotter paper LSD came "Windowpane" (AKA "Clearlight"), which contained LSD inside a thin gelatin square a quarter of an inch (6 mm) across.[127] LSD has been sold under a wide variety of often short-lived and regionally restricted street names including Acid, Trips, Uncle Sid, Blotter, Lucy, Alice and doses, as well as names that reflect the designs on the sheets of blotter paper.[129][130] Authorities have encountered the drug in other forms—including powder or crystal, and capsule.[131]
Modern distribution
LSD manufacturers and traffickers in the United States can be categorized into two groups: A few large-scale producers, and an equally limited number of small, clandestine chemists, consisting of independent producers who, operating on a comparatively limited scale, can be found throughout the country.[132] As a group, independent producers are of less concern to the Drug Enforcement Administration than the larger groups because their product reaches only local markets.[133]
Many LSD dealers and chemists describe a religious or humanitarian purpose that motivates their illicit activity. Nicholas Schou's book Orange Sunshine: The Brotherhood of Eternal Love and Its Quest to Spread Peace, Love, and Acid to the World describes one such group, the Brotherhood of Eternal Love. The group was a major American LSD trafficking group in the late 1960s and early 1970s.[134]
In the second half of the 20th century, dealers and chemists loosely associated with the Grateful Dead like Owsley Stanley, Nicholas Sand, Karen Horning, Sarah Maltzer, "Dealer McDope," and Leonard Pickard played an essential role in distributing LSD.[93]
Mimics
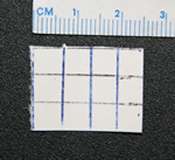
Since 2005, law enforcement in the United States and elsewhere has seized several chemicals and combinations of chemicals in blotter paper which were sold as LSD mimics, including DOB,[135][136] a mixture of DOC and DOI,[137] 25I-NBOMe,[138] and a mixture of DOC and DOB.[139] Street users of LSD are often under the impression that blotter paper which is actively hallucinogenic can only be LSD because that is the only chemical with low enough doses to fit on a small square of blotter paper. While it is true that LSD requires lower doses than most other hallucinogens, blotter paper is capable of absorbing a much larger amount of material. The DEA performed a chromatographic analysis of blotter paper containing 2C-C which showed that the paper contained a much greater concentration of the active chemical than typical LSD doses, although the exact quantity was not determined.[140] Blotter LSD mimics can have relatively small dose squares; a sample of blotter paper containing DOC seized by Concord, California police had dose markings approximately 6 mm apart.[141] Several deaths have been attributed to 25I-NBOMe.[142][143][144][145]
Research
A number of organizations—including the Beckley Foundation, MAPS, Heffter Research Institute and the Albert Hofmann Foundation—exist to fund, encourage and coordinate research into the medicinal and spiritual uses of LSD and related psychedelics.[146] New clinical LSD experiments in humans started in 2009 for the first time in 35 years.[147] As it is illegal in many areas of the world, potential medical uses are difficult to study.[17]
In 2001 the United States Drug Enforcement Administration stated that LSD "produces no aphrodisiac effects, does not increase creativity, has no lasting positive effect in treating alcoholics or criminals, does not produce a 'model psychosis', and does not generate immediate personality change."[148] More recently, experimental uses of LSD have included the treatment of alcoholism[149] and pain and cluster headache relief.[150]
Psychedelic therapy
In the 1950s and 1960s LSD was used in psychiatry to enhance psychotherapy known as psychedelic therapy. Some psychiatrists believed LSD was especially useful at helping patients to "unblock" repressed subconscious material through other psychotherapeutic methods,[151] and also for treating alcoholism.[152][153] One study concluded, "The root of the therapeutic value of the LSD experience is its potential for producing self-acceptance and self-surrender,"[154] presumably by forcing the user to face issues and problems in that individual's psyche.
Two recent reviews concluded that conclusions drawn from most of these early trials are unreliable due to serious methodological flaws. These include the absence of adequate control groups, lack of followup, and vague criteria for therapeutic outcome. In many cases studies failed to convincingly demonstrate whether the drug or the therapeutic interaction was responsible for any beneficial effects.[155][156]
In recent years organizations like the Multidisciplinary Association for Psychedelic Studies have renewed clinical research of LSD.[157]
Other uses
In the 1950s and 1960s, some psychiatrists (e.g. Oscar Janiger) explored the potential effect of LSD on creativity. Experimental studies attempted to measure the effect of LSD on creative activity and aesthetic appreciation.[158][159][160][161]
Since 2008 there has been ongoing research into using LSD to alleviate anxiety for terminally ill cancer patients coping with their impending deaths.[162][163]
A 2012 meta-analysis found evidence that a single dose of LSD in conjunction with various alcoholism treatment programs was associated with a decrease in alcohol abuse, lasting for several months, but no effect was seen at one year. Adverse events included seizure, moderate confusion and agitation, nausea, vomiting, and acting in a bizarre fashion.[164]
LSD has been used as a treatment for cluster headaches with positive results in some small studies.[150][165]
See also
- Urban legends about LSD
- History of lysergic acid diethylamide
- LSD art
- Psychedelic therapy
- Albert Hofmann
- Timothy Leary
- Owsley Stanley
References
- ↑ "Definition of "amide"". Collins English Dictionary. Retrieved January 31, 2015.
- ↑ "American Heritage Dictionary Entry: amide". Ahdictionary.com. Retrieved January 31, 2015.
- ↑ "amide - definition of amide in English from the Oxford dictionary". Oxforddictionaries.com. Retrieved January 31, 2015.
- ↑ Halpern, John H.; Suzuki, Joji; Huertas,, Pedro E.; Passie, Torsten (June 7, 2014). Price, Lawrence H.; Stolerman, Ian P., eds. Encyclopedia of Psychopharmacology A Springer Live Reference. Heidelberg, Germany: Springer-Verlag Berlin Heidelberg. pp. 1–5. ISBN 978-3-642-27772-6. Retrieved April 24, 2015.
Hallucinogen abuse and dependence are known complications resulting from the illicit use of drugs in this category, such as LSD and psilocybin. Users do not experience withdrawal symptoms, but the general criteria for substance abuse and dependence otherwise apply. Dependence is estimated in approximately 2 % of recent-onset users in the United States.
- 1 2 3 Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 15: Reinforcement and Addictive Disorders". In Sydor A, Brown RY. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. p. 375. ISBN 9780071481274.
Several other classes of drugs are categorized as drugs of abuse but rarely produce compulsive use. These include psychedelic agents, such as lysergic acid diethylamide (LSD), which are used for their ability to produce perceptual distortions at low and moderate doses. The use of these drugs is associated with the rapid development of tolerance and the absence of positive reinforcement (Chapter 6). Partial agonist effects at 5HT2A receptors are implicated in the psychedelic actions of LSD and related hallucinogens. 3,4-Methylenedioxymethamphetamine (MDMA), commonly called ecstasy, is an amphetamine derivative. It produces a combination of psychostimulant-like and weak LSD-like effects at low doses. Unlike LSD, MDMA is reinforcing—most likely because of its interactions with dopamine systems—and accordingly is subject to compulsive abuse. The weak psychedelic effects of MDMA appear to result from its amphetamine-like actions on the serotonin reuptake transporter, by means of which it causes transporter-dependent serotonin efflux. MDMA has been proven to produce lesions of serotonin neurons in animals and humans.
- 1 2 3 Aghajanian GK, Bing OH (1964). "Persistence of lysergic acid diethylamide in the plasma of human subjects" (PDF). Clinical Pharmacology and Therapeutics. 5: 611–614. PMID 14209776. Retrieved September 17, 2009.
- 1 2 Papac DI, Foltz RL (May–June 1990). "Measurement of lysergic acid diethylamide (LSD) in human plasma by gas chromatography/negative ion chemical ionization mass spectrometry" (PDF). Journal of Analytical Toxicology. 14 (3): 189–190. doi:10.1093/jat/14.3.189. PMID 2374410. Retrieved September 17, 2009.
- 1 2 3 4 "What are hallucinogens?". National Institute of Drug Abuse. January 2016. Retrieved April 24, 2016.
- 1 2 Lüscher C, Ungless MA (November 2006). "The Mechanistic Classification of Addictive Drugs". PLoS Med. 3 (11): e437. doi:10.1371/journal.pmed.0030437. PMC 1635740
 . PMID 17105338.
. PMID 17105338. - 1 2 Passie T, Halpern JH, Stichtenoth DO, Emrich HM, Hintzen A (2008). "The Pharmacology of Lysergic Acid Diethylamide: a Review". CNS Neuroscience & Therapeutics. 14 (4): 295–314. doi:10.1111/j.1755-5949.2008.00059.x. PMID 19040555.
- 1 2 3 4 5 6 7 Alexander and Ann Shulgin. "LSD", in TiHKAL (Berkeley: Transform Press, 1997). ISBN 0-9630096-9-9.
- 1 2 Greiner T, Burch NR, Edelberg R (1958). "Psychopathology and psychophysiology of minimal LSD-25 dosage; a preliminary dosage-response spectrum". AMA Arch Neurol Psychiatry. 79 (2): 208–10. doi:10.1001/archneurpsyc.1958.02340020088016. PMID 13497365.
- 1 2 3 Hofmann, Albert. LSD—My Problem Child (McGraw-Hill, 1980). ISBN 0-07-029325-2.
- ↑ "Hallucinogenic effects of LSD discovered". The History Channel.
- ↑ Arthur Stoll and Albert Hofmann LSD Patent April 30, 1943 in Switzerland and March 23, 1948 in the United States.
- ↑ "LSD: cultural revolution and medical advances". Royal Society of Chemistry. Retrieved September 27, 2007.
- 1 2 Nutt, David J.; King, Leslie A.; Nichols, David E. (2013-08-01). "Effects of Schedule I drug laws on neuroscience research and treatment innovation". Nature Reviews Neuroscience. 14 (8): 577–585. doi:10.1038/nrn3530. ISSN 1471-003X.
- ↑ "Scientists study possible health benefits of LSD and ecstasy | Science | The Guardian". July 23, 2016. Archived from the original on July 23, 2016. Retrieved 2016-07-23.
- ↑ "DrugFacts: Hallucinogens - LSD, Peyote, Psilocybin, and PCP". National Institute on Drug Abuse. December 2014. Retrieved February 17, 2015.
- ↑ Alcohol and Drugs in North America: A Historical Encyclopedia, by David M. Fahey and Jon S. Miller, Editors, ISBN 978-1-59884-478-8, page 375
- ↑ San Francisco Chronicle September 20, 1966 Page One
- ↑ Grof, Stanislav; Joan Halifax Grof (1979). Realms of the Human Unconscious (Observations from LSD Research). London: Souvenir Press (E & A) Ltd. pp. 13–14. ISBN 0-285-64882-9.
- ↑ NIDA InfoFacts: Hallucinogens - LSD, Peyote, Psilocybin, and PCP The National Institute on Drug Abuse (NIDA). Revised 06/09
- ↑ Schiff PL (2006). "Ergot and its alkaloids". American journal of pharmaceutical education. 70 (5): 98. PMID 17149427.
- 1 2 Rogge, Timothy (21 May 2014), Substance use - LSD, MedlinePlus, U.S. National Library of Medicine, retrieved 14 July 2016
- ↑ CESAR (29 October 2013), LSD, Center for Substance Abuse Research, University of Maryland, retrieved 14 July 2016
- 1 2 Linton Harriet B.; Langs Robert J. (1962). "Subjective Reactions to Lysergic Acid Diethylamide (LSD-25)" (PDF). Arch. Gen. Psychiatry. 6 (5): 352–68. doi:10.1001/archpsyc.1962.01710230020003.
- ↑ Albert Hofmann. "5. From Remedy to Inebriant". LSD: My Problem Child.
...taste of metal on the palate.
- ↑ Katz MM, Waskow IE, Olsson J (1968). "Characterizing the psychological state produced by LSD". J Abnorm Psychol. 73 (1): 1–14. doi:10.1037/h0020114. PMID 5639999.
- ↑ See, e.g., Gerald Oster's article "Moiré patterns and visual hallucinations". Psychedelic Rev. No. 7 (1966): 33–40.
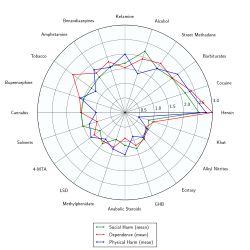
- ↑ Nutt, D; King, LA; Saulsbury, W; Blakemore, C (24 March 2007). "Development of a rational scale to assess the harm of drugs of potential misuse.". Lancet (London, England). 369 (9566): 1047–53. PMID 17382831.
- 1 2 Murray RM, Paparelli A, Morrison PD, Marconi A, Di Forti M (October 2013), "What can we learn about schizophrenia from studying the human model, drug-induced psychosis?", American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 162 (7, Special Issue: Identifying the Origins of Mental Illness: A Festschrift in Honor of Ming T. Tsuang): 661–670, doi:10.1002/ajmg.b.32177
- ↑ "Is Military Research Hazardous to Veterans Health? Lessons Spanning Half A Century, part F. HALLUCINOGENS". December 8, 1994 John D. Rockefeller IV, West Virginia: 103rd Congress, 2nd Session-S. Prt. 103-97; Staff Report prepared for the committee on veterans' affairs. December 8, 1994.
- ↑ Middlefell R (March 1967). "The effects of LSD on body sway suggestibility in a group of hospital patients" (PDF). Br J Psychiatry. 113 (496): 277–80. doi:10.1192/bjp.113.496.277. PMID 6029626.
- ↑ Sjoberg BM, Hollister LE (November 1965). "The effects of psychotomimetic drugs on primary suggestibility". Psychopharmacologia. 8 (4): 251–62. doi:10.1007/BF00407857. PMID 5885648.
- 1 2 Lerner AG, Gelkopf M, Skladman I, Oyffe I, Finkel B, Sigal M, Weizman A (2002). "Flashback and Hallucinogen Persisting Perception Disorder: Clinical aspects and pharmacological treatment approach". The Israel Journal of Psychiatry and Related Sciences. 39 (2): 92–9. PMID 12227234.
- ↑ Halpern JH, Pope HG (2003). "Hallucinogen persisting perception disorder: What do we know after 50 years?". Drug and Alcohol Dependence. 69 (2): 109–19. doi:10.1016/S0376-8716(02)00306-X. PMID 12609692.
- 1 2 Halpern, J (2003). "Hallucinogen persisting perception disorder: what do we know after 50 years?". Drug and Alcohol Dependence. 69 (2): 109–119. doi:10.1016/S0376-8716(02)00306-X. ISSN 0376-8716. PMID 12609692.
- 1 2 Blumenfield M (1971). "Flashback phenomena in basic trainees who enter the US Air Force". Military Medicine. 136 (1): 39–41. PMID 5005369.
- ↑ Hemsley, D.R.; Ward, E.S. (1985). "Individual differences in reaction to the abuse of LSD". Personality and Individual Differences. 6 (4): 515–517. doi:10.1016/0191-8869(85)90148-5. ISSN 0191-8869.
- ↑ Mark J. Russ; James M. Gold (1987). "LSD-Like Flashbacks Associated with ECT". Convulsive therapy. 3 (4): 296–301. PMID 11940932.
- ↑ R.D. Alarcon; W.A. Dickinson; H.H. Dohn (April 1982). "Flashback phenomena. Clinical and diagnostic dilemmas". The Journal of Nervous and Mental Disease. 170 (4): 217–223. doi:10.1097/00005053-198204000-00006. PMID 7062008.
- 1 2 Naditch MP, Fenwick S (1977). "LSD flashbacks and ego functioning". Journal of Abnormal Psychology. 86 (4): 352–9. doi:10.1037/0021-843X.86.4.352. PMID 757972.
- ↑ Frankel, Fred H. (1994). "The Concept of Flashbacks in Historical Perspective". International Journal of Clinical and Experimental Hypnosis. 42 (4): 321–336. doi:10.1080/00207149408409362. ISSN 0020-7144.
- ↑ Matefy, Robert E.; Hayes, Carla; Hirsch, Jerrold (1979). "Psychedelic drug flashbacks: Attentional deficits?". Journal of Abnormal Psychology. 88 (2): 212–215. doi:10.1037/0021-843X.88.2.212. ISSN 0021-843X.
- ↑ R. McGee (May 1984). "Flashbacks and memory phenomena. A comment on "Flashback phenomena--clinical and diagnostic dilemmas"". The Journal of Nervous and Mental Disease. 172 (5): 273–278. doi:10.1097/00005053-198405000-00004. PMID 6716092.
- ↑ R.J. Strassman (October 1984). "Adverse reactions to psychedelic drugs. A review of the literature". The Journal of Nervous and Mental Disease. 172 (10): 577–595. doi:10.1097/00005053-198410000-00001. PMID 6384428.
- ↑ Edward, Brecher. "The Consumers Union Report on Licit and Illicit Drugs (1971): Chapter 51".
- ↑ Abraham HD, Aldridge AM (1993). "Adverse consequences of lysergic acid diethylamide". Addiction (Abingdon, England). 88 (10): 1327–34. doi:10.1111/j.1360-0443.1993.tb02018.x. PMID 8251869.
- ↑ Abraham HD, Aldridge AM (1993). "Adverse consequences of lysergic acid diethylamide". Addiction. 88 (10): 1327–34. doi:10.1111/j.1360-0443.1993.tb02018.x. PMID 8251869.
- ↑ Halpern JH, Pope HG (2003). "Hallucinogen persisting perception disorder: what do we know after 50 years?". Drug Alcohol Depend. 69 (2): 109–19. doi:10.1016/S0376-8716(02)00306-X. PMID 12609692.
- ↑ Halpern JH (2003). "Hallucinogens: an update". Curr Psychiatry Rep. 5 (5): 347–54. doi:10.1007/s11920-003-0067-4. PMID 13678554.
- ↑ Li JH, Lin LF (November 1998). "Genetic toxicology of abused drugs: a brief review.". Mutagenesis. 13 (6): 557–565. doi:10.1093/mutage/13.6.557. PMID 9862186.
- ↑ "Gross behavioural changes in monkeys following administration of LSD-25, and development of tolerance to LSD-25 – Springer". Psychopharmacologia. 6: 303–306. doi:10.1007/BF00413161. Retrieved September 25, 2014.
- ↑ Wolbach AB, Isbell H, Miner EJ (1962). "Cross tolerance between mescaline and LSD-25, with a comparison of the mescaline and LSD reactions". Psychopharmacologia. 3: 1–14. doi:10.1007/BF00413101. PMID 14007904.
- ↑ Isbell H, Wolbach AB, Wikler A, Miner EJ (1961). "Cross Tolerance between LSD and Psilocybin". Psychopharmacologia. 2 (3): 147–59. doi:10.1007/BF00407974. PMID 13717955.
- 1 2 3 Nichols DE (2004). "Hallucinogens". Pharmacology & Therapeutics. 101 (2): 131–81. doi:10.1016/j.pharmthera.2003.11.002. PMID 14761703.
- ↑ McKenna DJ, Nazarali AJ, Himeno A, Saavedra JM (1989). "Chronic treatment with (+/-)DOI, a psychotomimetic 5-HT2 agonist, downregulates 5-HT2 receptors in rat brain". Neuropsychopharmacology. 2 (1): 81–87. doi:10.1016/0893-133X(89)90010-9. PMID 2803482.
- ↑ Buckholtz NS, Zhou DF, Freedman DX, Potter WZ (1990). "Lysergic acid diethylamide (LSD) administration selectively downregulates serotonin2 receptors in rat brain". Neuropsychopharmacology. 3 (2): 137–148. PMID 1969270.
- 1 2 3 LSD Toxicity Treatment & Management~treatment at eMedicine
- ↑ Klock, John C.; Boerner, Udo; Becker, Charles E. (1974). "Coma, Hyperthermia and Bleeding Associated with Massive LSD Overdose: A Report of Eight Cases". The Western Journal of Medicine. 120 (3): 183–8. PMC 1129381
 . PMID 4816396.
. PMID 4816396. - ↑ Marona-Lewicka D, Thisted RA, Nichols DE (2005). "Distinct temporal phases in the behavioral pharmacology of LSD: Dopamine D2 receptor-mediated effects in the rat and implications for psychosis". Psychopharmacology. 180 (3): 427–435. doi:10.1007/s00213-005-2183-9. PMID 15723230.
- ↑ Nichols, David (November 2012). "The End of a Chemistry Era... Dave Nichols Closes Shop". Retrieved September 24, 2013.
- ↑ "PDSP database". Archived from the original on May 17, 2013. Retrieved June 28, 2013.
- ↑ Nelson DL (February 2004). "5-HT5 receptors". Current drug targets. CNS and neurological disorders. 3 (1): 53–8. doi:10.2174/1568007043482606. PMID 14965244.
- ↑ Moreno JL, et al. (2011). "Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists". Neurosci Lett. 76 (3): 76–79. doi:10.1016/j.neulet.2011.01.046. PMC 3064746
 . PMID 21276828.
. PMID 21276828. - ↑ Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB (June 27, 2006). "Functional Selectivity and Classical Concepts of Quantitative Pharmacology". JPET. 320 (1): 1–13. doi:10.1124/jpet.106.104463. PMID 16803859.
- ↑ BilZ0r. "The Neuropharmacology of Hallucinogens: a technical overview". Erowid, v3.1 (August 2005).
- ↑ Svenningsson P, Nairn AC, Greengard P (2005). "DARPP-32 mediates the actions of multiple drugs of abuse". AAPS Journal. 7 (2): E353–E360. doi:10.1208/aapsj070235. PMC 2750972
 . PMID 16353915.
. PMID 16353915. - ↑ Borroto-Escuela DO, et al. (2014). "Hallucinogenic 5-HT2AR agonists LSD and DOI enhance dopamine D2R protomer recognition and signaling of D2-5-HT2A heteroreceptor complexes.". Biochem Biophys Res Commun. 278 (1): 278–84. doi:10.1016/j.bbrc.2013.11.104. PMID 24309097.
- ↑ Monte AP, Marona-Lewicka D, Kanthasamy A, Sanders-Bush E, Nichols DE (March 1995). "Stereoselective LSD-like activity in a series of d-lysergic acid amides of (R)- and (S)-2-aminoalkanes". Journal of Medicinal Chemistry. 38 (6): 958–66. doi:10.1021/jm00006a015. PMID 7699712.
- ↑ Nichols DE, Frescas S, Marona-Lewicka D, Kurrasch-Orbaugh DM (September 2002). "Lysergamides of isomeric 2,4-dimethylazetidines map the binding orientation of the diethylamide moiety in the potent hallucinogenic agent N,N-diethyllysergamide (LSD)". Journal of Medicinal Chemistry. 45 (19): 4344–9. doi:10.1021/jm020153s. PMID 12213075.
- ↑ "Erowid Morning Glory Vaults : Extraction of LSA (Method #1)". erowid.org. Retrieved September 25, 2014.
- ↑ Kornfeld, Edmund C.; Fornefeld, E. J.; Kline, G. Bruce; Mann, Marjorie J.; Morrison, Dwight E.; Jones, Reuben G.; Woodward, R. B. (1956). "The Total Synthesis of Lysergic Acid". Journal of the American Chemical Society. 78 (13): 3087–3114. doi:10.1021/ja01594a039.
- ↑ Inuki S, Oishi S, Fujii N, Ohno H (2008). "Total Synthesis of (±)-Lysergic Acid, Lysergol, and Isolysergol by Palladium-Catalyzed Domino Cyclization of Amino Allenes Bearing a Bromoindolyl Group". Organic Letters. 10 (22): 5239–5242. doi:10.1021/ol8022648. PMID 18956869.
- ↑ Stoll, W.A. (1947). "Ein neues, in sehr kleinen Mengen wirsames Phantastikum". Arch. Neurol. Schweiz. 60: 483.
- 1 2 Erowid & Eduardo Hidalgo, Energy Control (Spain) (2009). "LSD Samples Analysis". Erowid. Retrieved February 8, 2010.
- 1 2 Henderson, Leigh A.; Glass, William J. (1994). LSD: Still with us after all these years. San Francisco: Jossey-Bass. ISBN 978-0-7879-4379-0.
- ↑ Fire & Earth Erowid (2003). "LSD Analysis – Do we know what's in street acid?". Erowid. Retrieved February 8, 2010.
- ↑ Li Z, McNally AJ, Wang H, Salamone SJ (October 1998). "Stability study of LSD under various storage conditions". J. Anal. Toxicol. 22 (6): 520–5. doi:10.1093/jat/22.6.520. PMID 9788528.
- ↑ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 871–874.
- ↑ Papac DI, Foltz RL (1990). "Measurement of lysergic acid diethylamide (LSD) in human plasma by gas chromatography/negative ion chemical ionization mass spectrometry". J Anal Toxicol. 14: 189–90. doi:10.1093/jat/14.3.189. PMID 2374410.
- ↑ Hofmann 1980, p. 15
- ↑ Albert Hofmann; translated from the original German (LSD Ganz Persönlich) by J. Ott. MAPS-Volume 6, Number 69, Summer 1969
- ↑ Nichols, David (May 24, 2003). "Hypothesis on Albert Hofmann's Famous 1943 "Bicycle Day"". Hofmann Foundation. Retrieved September 27, 2007.
- ↑ Albert Hofmann. "LSD My Problem Child". Retrieved April 19, 2010.
- ↑ Hofmann, Albert. "History Of LSD". Archived from the original on September 4, 2007. Retrieved September 27, 2007.
- ↑ DEA Public Affairs (November 16, 2001). "LSD: The Drug". Web.petabox.bibalex.org. Archived from the original on August 23, 2013. Retrieved November 27, 2010.
- ↑ "Brecher, Edward M; et al. (1972). "How LSD was popularized". Consumer Reports/Drug Library". Druglibrary.org. Retrieved June 20, 2012.
- ↑ Anne Applebaum, "Did The Death Of Communism Take Koestler And Other Literary Figures With It?", The Huffington Post, January 26, 2010.
- ↑ "Out-Of-Sight! SMiLE Timeline". Archived from the original on February 1, 2010. Retrieved October 30, 2011.
- ↑ L. R. Veysey, The Communal Experience: Anarchist and Mystical Communities in Twentieth-Century America (Chicago IL, University of Chicago Press, 1978), ISBN 0-226-85458-2, p. 437.
- 1 2 3 Jarnow, Jesse (2016). Heads: A Biography of Psychedelic America. Da Capo Press. ISBN 9780306822551.
- ↑ United States Congress (October 24, 1968). "Staggers-Dodd Bill, Public Law 90-639" (PDF). Retrieved September 8, 2009.
- ↑ Gasser, Peter (1994). "Psycholytic Therapy with MDMA and LSD in Switzerland". Retrieved September 8, 2009.
- ↑ J. DeRogatis, Turn On Your Mind: Four Decades of Great Psychedelic Rock (Milwaukie, Michigan: Hal Leonard, 2003), ISBN 0-634-05548-8, pp. 8–9.
- ↑ Gilliland, John (1969). "Show 41 – The Acid Test: Psychedelics and a sub-culture emerge in San Francisco. [Part 1] : UNT Digital Library" (audio). Pop Chronicles. Digital.library.unt.edu. Retrieved May 6, 2011.
- ↑ M. Hicks, Sixties Rock: Garage, Psychedelic, and Other Satisfactions Music in American Life (Chicago, IL: University of Illinois Press, 2000), ISBN 0-252-06915-3, p. 60.
- ↑ J. Mann, Turn on and Tune in: Psychedelics, Narcotics and Euphoriants (Royal Society of Chemistry, 2009), ISBN 1-84755-909-3, p. 87.
- ↑ Joshua Clark Davis, The Business of Getting High: Head Shops, Countercultural Capitalism, and the Marijuana Legalization Movement. The Sixties: A Journal of Politics, Culture and Society, Summer 2015. Free full text
- ↑ R. Rubin and J. P. Melnick, Immigration and American Popular Culture: an Introduction (New York, NY: New York University Press, 2007), ISBN 0-8147-7552-7, pp. 162–4.
- ↑ P. Prown, H. P. Newquist and J. F. Eiche, Legends of Rock Guitar: the Essential Reference of Rock's Greatest Guitarists (London: Hal Leonard Corporation, 1997), ISBN 0-7935-4042-9, p. 48.
- ↑ S. Borthwick and R. Moy, Popular Music Genres: an Introduction (Edinburgh: Edinburgh University Press, 2004), ISBN 0-7486-1745-0, pp. 52–4.
- ↑ J. DeRogatis, Turn On Your Mind: Four Decades of Great Psychedelic Rock (Milwaukie, Michigan: Hal Leonard, 2003), ISBN 0-634-05548-8, p. 230.
- ↑ Richie Unterberger, Samb Hicks, Jennifer Dempsey, "Music USA: the rough guide" (Rough Guides, 1999), ISBN 1-85828-421-X, p. 391.
- ↑ G. St. John, Rave Culture and Religion (Abingdon: Routledge, 2004), ISBN 0-415-31449-6, p. 52.
- ↑ M. Campbell, Popular Music in America: And the Beat Goes on (Boston, MA: Cengage Learning, 3rd edn., 2008), ISBN 0-495-50530-7, pp. 212–3.
- ↑ UN Convention on Psychotropic Substances, 1971 Final act of the United Nations Conference
- 1 2 Poisons Standard July 2016 Comlaw.gov.au
- ↑ Misuse of Drugs Act 1981 (2015) Slp.wa.gov.au
- ↑ Canadian government (1996). "Controlled Drugs and Substances Act". Justice Laws. Canadian Department of Justice. Archived from the original on December 15, 2013. Retrieved December 15, 2013.
- ↑ Drugs and the law: Report of the inquiry into the Misuse of Drugs Act 1971 London: Police Foundation, 2000, Runciman Report
- ↑ After the War on Drugs: Blueprint for Regulation Transform Drug Policy Foundation 2009
- ↑ From "Archived copy". Archived from the original on October 5, 2009. Retrieved February 5, 2016.: LSD is a Schedule I substance under the Controlled Substances Act.
- ↑ LSD Toxicity at eMedicine
- ↑ Neal v. United States, U.S. 284 (1996). , originating from U.S. v. Neal, 46 F.3d 1405 (7th Cir. 1995)
- ↑ Ley de Narcomenudeo, El Pensador (Spanish), 17 October 2009
- ↑ Mexico: The Law Against Small-Scale Drug Dealing. A Doubtful Venture, Jorge Hernández Tinajero & Carlos Zamudio Angles, Series on Legislative Reform of Drug Policies Nr. 3, November 2009
- 1 2 Parliament of the Czech Republic (1998), Explanatory Report to Act No. 112/1998 Coll., which amends the Act No. 140/1961 Coll., the Criminal Code, and the Act No. 200/1990 Coll., on misdemeanors (in Czech), Prague "Podle čl. 36 Jednotné úmluvy o omamných látkách ze dne 31. března 1961 (č. 47/1965 Sb.) se signatáři zavazují k trestnímu postihu tam uvedených forem nakládání s drogami včetně jejich držby. Návrh upouští od dosavadní beztrestnosti držby omamných a psychotropních látek a jedů pro svoji potřebu. Dosavadní beztrestnost totiž eliminuje v řadě případů možnost postihu dealerů a distributorů drog."
- ↑ Supreme Court of the Czech Republic (25 February 2012), 6 Tdo 156/2010 [NS 7078/2010]
- ↑ Government of the Czech Republic (2009), Regulation No. 467/2009 Coll., which defines for the purposes of the Criminal Code what is to be considered larger than small amount of narcotic and psychoactive substances and poisons (in Czech), Prague
- 1 2 "La nueva tabla para consumo de drogas es una guía para jueces". Archived from the original on 22 June 2013.
- ↑ "Dosis máximas de droga para consumo ya están vigentes" at El Comercio.com.
- ↑ "Ecuador: Aprueban tenencia de drogas para consumo" at El Nuevo Herald
- ↑ "Ecuador could regulate the drug industry".
- 1 2 DEA (2007). "LSD Manufacture – Illegal LSD Production". LSD in the United States. U.S. Department of Justice Drug Enforcement Administration. Archived from the original on August 29, 2007.
- 1 2 3 Stafford, Peter (1992). "Chapter 1 – The LSD Family". Psychedelics Encyclopaedia (Third Expanded ed.). Ronin Publishing Inc. p. 62. ISBN 0-914171-51-8.
- 1 2 3 Laing, Richard R.; Barry L. Beyerstein; Jay A. Siegel (2003). "Chapter 2.2 – Forms of the Drug". Hallucinogens: A Forensic Drug Handbook. Academic Press. pp. 39–41. ISBN 0-12-433951-4.
- ↑ Honig, David. Frequently Asked Questions via Erowid
- ↑ "Street Terms: Drugs and the Drug Trade". Office of National Drug Control Policy. April 5, 2005. Retrieved January 31, 2007.
- ↑ DEA (2008). "Photo Library (page 2)". US Drug Enforcement Administration. Archived from the original on June 23, 2008. Retrieved June 27, 2008.
- ↑ ^ Maclean, J.R.; Macdonald, D.C.; Ogden, F.; Wilby, E., "LSD-25 and mescaline as therapeutic adjuvants." In: Abramson, H., Ed., The Use of LSD in Psychotherapy and Alcoholism, Bobbs-Merrill: New York, 1967, pp. 407–426; Ditman, K.S.; Bailey, J.J., "Evaluating LSD as a psychotherapeutic agent," pp.74–80; Hoffer, A., "A program for the treatment of alcoholism: LSD, malvaria, and nicotinic acid," pp. 353–402.
- ↑ ^ LSD: The Drug
- ↑ Schou, Nicholas (2010). Orange Sunshine: The Brotherhood of Eternal Love and Its Quest to Spread Peace, Love, and Acid to the World. Thomas Dunne Books.
- ↑ United States Drug Enforcement Administration (October 2005). "LSD BLOTTER ACID MIMIC CONTAINING 4-BROMO-2, 5-DIMETHOXY-AMPHETAMINE (DOB) SEIZED NEAR BURNS, OREGON" (PDF). Microgram Bulletin. 38 (10). Retrieved August 20, 2009.
- ↑ United States Drug Enforcement Administration (November 2006). ""PURPLE" (COUGH SYRUP CONTAINING CODEINE AND OXYCODONE) IN WILKINSBURG, PENNSYLVANIA" (PDF). Microgram Bulletin. 39 (11). Retrieved August 20, 2009.
- ↑ United States Drug Enforcement Administration (March 2008). "UNUSUAL "RICE KRISPIE TREAT"-LIKE BALLS CONTAINING PSILOCYBE MUSHROOM PARTS IN WARREN COUNTY, MISSOURI" (PDF). Microgram Bulletin. 41 (3). Retrieved August 20, 2009.
- ↑ Iversen, Les (May 29, 2013). "Temporary Class Drug Order Report on 5-6APB and NBOMe compounds" (PDF). Advisory Council on the Misuse of Drugs. Gov.Uk. p. 14. Retrieved June 16, 2013.
- ↑ United States Drug Enforcement Administration (March 2009). ""SPICE" – PLANT MATERIAL(S) LACED WITH SYNTHETIC CANNABINOIDS OR CANNABINOID MIMICKING COMPOUNDS" (PDF). Microgram Bulletin. 42 (3). Retrieved August 20, 2009.
- ↑ United States Drug Enforcement Administration (November 2005). "BULK MARIJUANA IN HAZARDOUS PACKAGING IN CHICAGO, ILLINOIS" (PDF). Microgram Bulletin. 38 (11). Retrieved August 20, 2009.
- ↑ United States Drug Enforcement Administration (December 2007). "SMALL HEROIN DISKS NEAR GREENSBORO, GEORGIA" (PDF). Microgram Bulletin. 40 (12). Retrieved August 20, 2009.
- ↑ Erowid. "25I-NBOMe (2C-I-NBOMe) Fatalities / Deaths". Drug Website. Erowid. Retrieved February 28, 2016.
- ↑ Hastings, Deborah (May 6, 2013). "New drug N-bomb hits the street, terrifying parents, troubling cops". New York Daily News. Retrieved May 7, 2013.
- ↑ Feehan, Conor (January 21, 2016). "Powerful N-Bomb drug - responsible for spate of deaths internationally - responsible for hospitalisation of six in Cork". Irish Independent. Retrieved January 22, 2016.
- ↑ Iversen, Les (May 29, 2013). "Temporary Class Drug Order Report on 5-6APB and NBOMe compounds" (PDF). Advisory Council on the Misuse of Drugs. Gov.Uk. Retrieved June 16, 2013.
- ↑ "The Albert Hofmann Foundation". Hofmann Foundation. Retrieved September 27, 2007.
- ↑ "LSD & Psilocybin-Assisted Therapy for Anxiety". Retrieved October 16, 2013.
- ↑ DEA Public Affairs (November 16, 2001). "DEA – Publications – LSD in the US – The Drug". Web.petabox.bibalex.org. Archived from the original on August 23, 2013. Retrieved November 27, 2010.
- ↑ Bogenschutz MP (2013). "Studying the effects of classic hallucinogens in the treatment of alcoholism: Rationale, methodology, and current research with psilocybin". Current drug abuse reviews. 6 (1): 17–29. doi:10.2174/15733998113099990002. PMID 23627783.
- 1 2 Passie T, Halpern JH, Stichtenoth DO, Emrich HM, Hintzen A (2008). "The pharmacology of lysergic acid diethylamide: A review". CNS Neuroscience & Therapeutics. 14 (4): 295–314. doi:10.1111/j.1755-5949.2008.00059.x. PMID 19040555.
- ↑ Cohen, S. (1959). The therapeutic potential of LSD-25. A Pharmacologic Approach to the Study of the Mind, p251–258.
- ↑ "Use of d-lysergic acid diethylamide in the treatment of alcoholism". Retrieved June 20, 2012.
- ↑ "Use of d-Lysergic Acid Diethylamide in the Treatment of Alcoholism". Hofmann.org. November 14, 2003. Retrieved February 22, 2012.
- ↑ Chwelos N, Blewett DB, Smith CM, Hoffer A (1959). "Use of d-lysergic acid diethylamide in the treatment of alcoholism". Quart. J. Stud. Alcohol. 20: 577–90. PMID 13810249.
- ↑ Vollenweider, F. X.; Kometer, M (2010). "The neurobiology of psychedelic drugs: Implications for the treatment of mood disorders". Nature Reviews Neuroscience. 11 (9): 642–51. doi:10.1038/nrn2884. PMID 20717121.
- ↑ Baumeister, D; Barnes, G; Giaroli, G; Tracy, D (2014). "Classical hallucinogens as antidepressants? A review of pharmacodynamics and putative clinical roles". Therapeutic Advances in Psychopharmacology. 4 (4): 156–69. doi:10.1177/2045125314527985. PMC 4104707
 . PMID 25083275.
. PMID 25083275. - ↑ "LSD-Assisted Psychotherapy". MAPS. Retrieved 3 October 2016.
- ↑ Sessa B (2008). "Is it time to revisit the role of psychedelic drugs in enhancing human creativity?". J Psychopharmacol. 22 (8): 821–7. doi:10.1177/0269881108091597. PMID 18562421.
- ↑ Janiger O, Dobkin de Rios M (1989). "LSD and creativity". J Psychoactive Drugs. 21 (1): 129–34. doi:10.1080/02791072.1989.10472150. PMID 2723891.
- ↑ Stafford, Peter G.; B. H. Golightly (1967). LSD, the problem-solving psychedelic. ASIN B0006BPSA0.
- ↑ McGlothlin W, Cohen S, McGlothlin MS (1967). "Long lasting effects of LSD on normals" (PDF). Archives of General Psychiatry. 17 (5): 521–532. doi:10.1001/archpsyc.1967.01730290009002. PMID 6054248.
- ↑ "Psychiater Gasser bricht sein Schweigen". July 28, 2009.
- ↑ LSD-Assisted Psychotherapy http://www.maps.org/research/psilo-lsd
- ↑ Krebs, T. S.; Johansen, P.-O. (2012). "Lysergic acid diethylamide (LSD) for alcoholism: meta-analysis of randomized controlled trials". Journal of Psychopharmacology. 26 (7): 994–1002. doi:10.1177/0269881112439253. PMID 22406913.
- ↑ Berlin pilot cluster headaches treatment with LSD study. LSD Alleviates 'Suicide Headaches' Archived January 10, 2012, at the Wayback Machine..
Further reading
- Bebergal, Peter, "Will Harvard drop acid again? Psychedelic research returns to Crimsonland", The Phoenix (Boston), June 2, 2008
- Passie T, Halpern JH, Stichtenoth DO, Emrich HM, Hintzen A (2008). "The pharmacology of lysergic acid diethylamide: a review". CNS Neuroscience & Therapeutics. 14 (4): 295–314. doi:10.1111/j.1755-5949.2008.00059.x. PMID 19040555.
External links
| Wikiquote has quotations related to: LSD |
| Wikimedia Commons has media related to LSD. |
| Wikinews has related news: Drug website surveys LSD users and culture |
- Drug Profiles: LSD European Monitoring Centre for Drugs and Drug Addiction
- LSD-25 at Erowid
- The Lycaeum Archive: LSD
- LSD entry in TiHKAL • info
- InfoFacts – Hallucinogens NIDA
- Scholarly bibliography on the histories of LSD use
- LSD Returns-For Psychotherapeutics (Scientific American Magazine article)
- U.S. National Library of Medicine: Drug Information Portal – Lysergic acid diethylamide
- My LSD Trip: a non-cop, non-hippie report of the unvarnished facts, by Robert Gannon, Popular Science Magazine, December 1967.
- WWW Psychedelic Bibliography, MAPS – large database of scientific publications on LSD and other psychedelics, fulltext PDFs
Documentaries
- Hofmann's Potion a documentary on the origins of LSD
- Power & Control LSD in The Sixties on YouTube, documentary film directed by Aron Ranen, 2006
- Inside LSD National Geographic Channel, 2009
.svg.png)