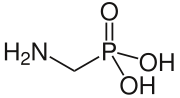
Aminomethylphosphonic acid
Not to be confused with AMPA.
 | |
| Names | |
|---|---|
| IUPAC name
(Aminomethyl)phosphonic acid | |
| Other names
Aminomethanephosphonic acid | |
| Identifiers | |
| 1066-51-9 | |
| 3D model (Jmol) | Interactive image |
| Abbreviations | AMPA; AMeP |
| ChEBI | CHEBI:28812 |
| ChemSpider | 13399 |
| ECHA InfoCard | 100.152.014 |
| PubChem | 14017 |
| UNII | 90825O5C1U |
| |
| |
| Properties | |
| CH6NO3P | |
| Molar mass | 111.04 g·mol−1 |
| Appearance | Solid |
| Melting point | 338 to 344 °C (640 to 651 °F; 611 to 617 K) |
| Acidity (pKa) | 0.4 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Aminomethylphosphonic acid (AMPA) is a weak organic acid with a phosphonic acid group. It is one of the primary degradation products of the herbicide glyphosate.[1] AMPA has low toxicity which is comparable to that of glyphosate and it is therefore considered to be of no greater toxicological concern than glyphosate itself.[2] AMPA has been shown to be broken down further by manganese oxide which occurs naturally in soil,[3] or to phosphoric acid via bacterial action [4][5] and ultimately to carbon dioxide and inorganic phosphate.[6]
References
- ↑ Environmental Fate of Glyphosate, Jeff Schuette, Department of Pesticide Regulation, California
- ↑ Pesticide Residues in Food - 1997, FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group
- ↑ K. A. Barrett and M. B. McBride. Oxidative Degradation of Glyphosate and Aminomethylphosphonate by Manganese Oxide. Environ. Sci. Technol., 2005, 39 (23), pp 9223–9228
- ↑ Pipke R, Amrhein N. (1988) Isolation and characterization of a mutant of Arthrobacter sp. strain GLP-1 which utilizes the herbicide glyphosate as its sole source of phosphorus and nitrogen. Applied and Environmental Microbiology 54(11): 2868-2870.
- ↑ Forlani G, Mangiagalli A, Nielsen E, Suardi CM. (1999) Degradation of the phosphonate herbicide glyphosate in soil: Evidence for a possible involvement of unculturable microorganisms. Soil Biology and Biochemistry 31: 991-997
- ↑ Backgrounder: Glyphosate does not degrade to phosphorous acid in the environment. Monsanto. 2005
External links
- Concentrations of Glyphosate, Its Degradation Product, Aminomethylphosphonic Acid, and Glufosinate in Ground- and Surface-Water, Rainfall, and Soil Samples Collected in the United States, 2001-06, United States Geological Survey
This article is issued from Wikipedia - version of the 7/2/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.