Benzvalene
 | |
| Names | |
|---|---|
| IUPAC name
tricyclo[3.1.0.02,6]hex-3-ene | |
| Identifiers | |
| 659-85-8 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 120239 |
| PubChem | 136470 |
| |
| |
| Properties | |
| C6H6 | |
| Molar mass | 78.11 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
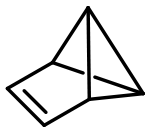
Benzvalene is an organic compound and one of several isomers of benzene.[1] It was first synthesized in 1971 by Thomas J. Katz et al.[2][3]
The 1971 synthesis consisted of treating cyclopentadiene with methyllithium in dimethyl ether and then with dichloromethane and methyllithium in diethyl ether at −45 °C. The hydrocarbon in solution was described as having an extraordinary mephitic odor. Due to the high steric strain present in benzvalene, the pure compound easily detonates, for example by scratching.
The compound converts to benzene with a chemical half-life of approximately 10 days. This symmetry-forbidden transition is believed to take place through a diradical intermediate.[4]
Polybenzvalene
Benzvalene can be polymerized in a ROMP process to polybenzvalene.[5] This polymer contains highly strained bicyclobutane rings which again makes it a sensitive material. The rings can be isomerized to 1,3-dienes and for this reason polybenzvalene has been investigated as a precursor to polyacetylene.
References
- ↑ Christl, M. (1981). "Benzvalene—Properties and Synthetic Potential". Angewandte Chemie International Edition in English. 20 (67): 529–521. doi:10.1002/anie.198105291.
- ↑ Katz, T. J.; Wang, E. J.; Acton, N. (1971). "Benzvalene synthesis". Journal of the American Chemical Society. 93 (15): 3782. doi:10.1021/ja00744a045.
- ↑ Katz, T. J.; Roth, R. J.; Acton, N.; Carnahan, E. J. (1999). "Synthesis of Benzvalene". The Journal of Organic Chemistry. 64 (20): 7663. doi:10.1021/jo990883g.
- ↑ Scott, Lawrence T.; Jones, Maitland. (1972). "Rearrangements and interconversions of compounds of the formula (CH)n". Chemical Reviews. 72 (2): 181. doi:10.1021/cr60276a004.
- ↑ Swager, T. M.; Dougherty, D. A.; Grubbs, R. H. (1988). "Strained rings as a source of unsaturation: polybenzvalene, a new soluble polyacetylene precursor". Journal of the American Chemical Society. 110 (9): 2973. doi:10.1021/ja00217a049.
External links
![]() Media related to Benzvalene at Wikimedia Commons
Media related to Benzvalene at Wikimedia Commons