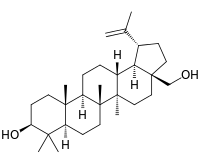
Betulin
 | |
| Names | |
|---|---|
| IUPAC name
Lup-20(29)-ene-3β,28-diol | |
| Identifiers | |
| 473-98-3 | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChEMBL | ChEMBL23236 |
| ChemSpider | 65272 |
| ECHA InfoCard | 100.006.797 |
| EC Number | 207-475-5 |
| KEGG | C08618 |
| PubChem | 72326 |
| |
| |
| Properties | |
| C30H50O2 | |
| Molar mass | 442.73 g·mol−1 |
| Melting point | 256 to 257 °C (493 to 495 °F; 529 to 530 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Betulin (lup-20(29)-ene-3β,28-diol) is an abundant, naturally occurring triterpene. It is commonly isolated from the bark of birch trees, where it forms up to 30% of the dry weight of the extractive,[1] and is found in birch sap as well.[2] The purpose of the compound in the bark is not known. It can be converted to betulinic acid (the alcohol group replaced by a carboxylic acid group), which is biologically more active than betulin itself.
History
Betulin was discovered in 1788 by German-Russian chemist Johann Tobias Lowitz.
Chemistry
Chemically, betulin is a triterpenoid of lupane structure. It has a pentacyclic ring structure, and hydroxyl groups in positions C3 and C28.
Biological activities
Recent clinical studies[3] have shown that betulin (3β-lup-20(29)-en-3,28-diol), was effective against a variety of tumors. Betulin causes some types of tumor cells to start a process of self-destruction called apoptosis and can slow the growth of several types of tumor cells.
Recent studies[4] have proved that betulin, inhibited the maturation of sterol regulatory element-binding protein (SREBPs). Inhibition of SREBP by betulin decreased the biosynthesis of cholesterol and fatty acid. In vivo, betulin ameliorated diet-induced obesity, decreased the lipid contents in serum and tissues, and increased insulin sensitivity. Furthermore, betulin reduced the size and improved the stability of atherosclerotic plaques.
Inonotus obliquus contains betulin,[5] Native Americans used red alder bark to treat poison oak, insect bites, and skin irritations. Blackfeet Indians used an infusion made from the bark of red alder to treat lymphatic disorders and tuberculosis.[6]
References
- ↑ Green, Brian; Bentley, Michael D.; Chung, Bong Y.; Lynch, Nicholas G.; Jensen, Bruce L. (2007), "Isolation of Betulin and Rearrangement to Allobetulin A Biomimetic Natural Product Synthesis", J. Chem. Educ., 84: 1985, doi:10.1021/ed084p1985.
- ↑ http://www2.publicationsduquebec.gouv.qc.ca/essences/arbre.php?id=98
- ↑ Alakurtti, S; Makela, T; Koskimies, S; Yli-Kauhaluoma, J (2006). "harmacological properties of the ubiquitous natural product betulin". Eur. J. Pharm. Sci. 29 (1): 1–13. doi:10.1016/j.ejps.2006.04.006. PMID 16716572.
- ↑ Tang, Jing-Jie; et al. (2011). "Inhibition of SREBP by a Small Molecule, Betulin, Improves Hyperlipidemia and Insulin Resistance and Reduces Atherosclerotic Plaques". j. cmet. 13 (1): 44–56. doi:10.1016/j.cmet.2010.12.004.
- ↑ Gao, Y; Xu, H; Lu, Z; Xu, Z (2009). "Quantitative determination of steroids in the fruiting bodies and submerged-cultured mycelia of Inonotus obliquus". Se pu. 27 (6): 745–9. PMID 20352924.
- ↑ Tilford, Gregory L. (1997), Edible and Medicinal Plants of the West, Missoula, MO: Mountain Press, ISBN 0-87842-359-1.