Bipolar electrochemistry
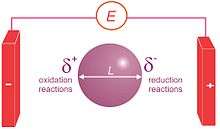
Bipolar electrochemistry is a phenomenon in electrochemistry based on the polarization of conducting objects in electric fields. Indeed, this polarization generates a potential difference between the two extremities of the substrate that is equal to the electric field value multiplied by the size of the object. If this potential difference is important enough, then redox reactions can be generated at the extremities of the object, oxidations will occur at one extremity coupled simultaneously to reductions at the other extremity.[1][2] In a simple experimental setup consisting of a platinum wire in a weighing boat containing a pH indicator solution, a 30 V voltage across two electrodes will cause water reduction at one end of the wire (the cathode) and a pH increase (OH− formation) and water oxidation at the anodic end and a pH decrease. The poles of the bipolar electrode also align themselves with the applied electric field.[3]
Utilisations
The phenomenon of bipolar electrochemistry is known for several decades and is used in industry in some electrolytic reactors. The interest of the scientific community for this concept seems to increase a lot since Martin Fleischmann and co-workers demonstrated that water splitting was possible using micrometer-sized bipolar electrodes.[4] Recently, several applications in such domains as synthesis of dissymmetrical micro- and nano-structures[5][6] analytical chemistry[7][8][9] material science,[10] microelectronics [11] and microobject propulsion[12][13] have been developed.
References
- ↑ G. Loget; A. Kuhn (2011). "Shaping and exploring the micro- and nanoworld using bipolar electrochemistry". Analytical and Bioanalytical Chemistry. 400 (6): 1691. doi:10.1007/s00216-011-4862-1.
- ↑ F. Mavré; R. K. Anand; D. R. Laws; K.-F. Chow; B.-Y. Chang; J. A. Crooks; R. M. Crooks (2010). "Feature Bipolar Electrodes: A Useful Tool for Concentration, Separation, and Detection of Analytes in Microelectrochemical Systems". Anal. Chem. 82 (21): 8766. doi:10.1021/ac101262v.
- ↑ Fosdick, S. E.; Knust, K. N.; Scida, K.; Crooks, R. M. (2013). "Bipolar Electrochemistry". Angew. Chem. Int. Ed. 52: 10438–10456. doi:10.1002/anie.201300947.
- ↑ M. Fleischmann; J. Ghoroghchian; D. Rolison; S. Pons (1986). "Electrochemical behavior of dispersions of spherical ultramicroelectrodes". J. Phys. Chem. 90 (23): 6392. doi:10.1021/j100281a065.
- ↑ G. Loget; V. Lapeyre; P. Garrigue; C. Warakulwit; J. Limtrakul; M.-H. Delville; A. Kuhn (2011). "Versatile Procedure for Synthesis of Janus-Type Carbon Tubes". Chem. Mater. 23 (10): 2595. doi:10.1021/cm2001573.
- ↑ C. Warakulwit; T. Nguyen; J. Majimel; M.-H. Delville; V. Lapeyre; P. Garrigue; V.Ravaine; J. Limtrakul; A. Kuhn (2008). "Dissymmetric Carbon Nanotubes by Bipolar Electrochemistry". Nano Lett. 8 (2): 500. Bibcode:2008NanoL...8..500W. doi:10.1021/nl072652s.
- ↑ K.-F. Chow; B.-Y. Chang; B. A. Zaccheo; F. Mavré; R. M. Crooks (2010). "A Sensing Platform Based on Electrodissolution of a Ag Bipolar Electrode". J. Am. Chem. Soc. 132 (27): 9228. doi:10.1021/ja103715u.
- ↑ Hlushkou D, Perdue RK, Dhopeshwarkar R, Crooks RM, Tallarek U (2009). "Electric field gradient focusing in microchannels with embedded bipolar electrode". Lab Chip. 9 (13): 1903. doi:10.1039/b822404h.
- ↑ Ulrich C, Andersson O, Nyholm L, Björefors F (2009). "Potential and Current Density Distributions at Electrodes Intended for Bipolar Patterning". Anal. Chem. 81: 453. doi:10.1021/ac801871c.
- ↑ Ramakrishnan S, Shannon C (2010). "Display of Solid-State Materials Using Bipolar Electrochemistry". Langmuir. 26 (7): 4602. doi:10.1021/la100292u.
- ↑ J. C. Bradley; H. M. Chen; J. Crawford; J. Eckert; K. Ernazarova; T. Kurzeja; M. Lin; M. McGee; W. Nadler; S. G. Stephens (1997). "Creating electrical contacts between metal particles using directed electrochemical growth". Nature. 389 (6648): 268. Bibcode:1997Natur.389..268B. doi:10.1038/38464.
- ↑ G. Loget; A. Kuhn (2010). "Propulsion of Microobjects by Dynamic Bipolar Self-Regeneration". J. Am. Chem. Soc. 132 (45): 15918. doi:10.1021/ja107644x.
- ↑ G. Loget; A. Kuhn (2011). "Electric field-induced chemical locomotion of conducting objects". Nature Communications. 2 (11): 535. Bibcode:2011NatCo...2E.535L. doi:10.1038/ncomms1550.