Carbometalation
Carbometalation is an organometallic reaction involving the nucleophilic addition to alkenes and alkynes of a diverse range of organometallic reagents such as organolithium compounds, organocopper compounds and Grignard reagents according to the following general alkyne scheme:
The addition can yield the cis or trans isomer and with unsymmetrical alkynes the organometallic compound can add in two different way thus control of regioselectivity is important.
In a follow-up step the sensitive metalalkenyl group is replaced by an electrophile E+.
Scope
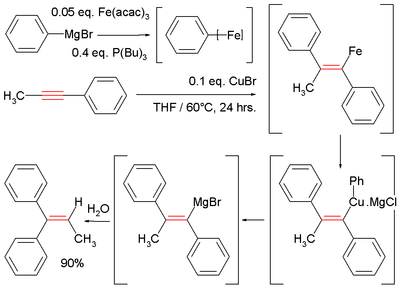
In one study methylphenylacetylene is reacted with phenylmagnesium bromide to a vinyl magnesium bromide which is quenched with water:[1][2]
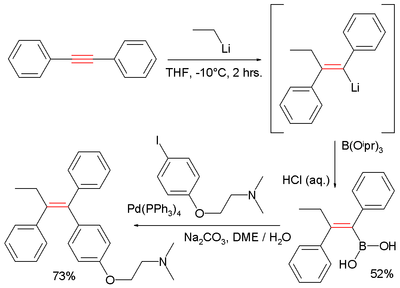
Another demonstration of this reaction type is an alternative route to tamoxifen starting from diphenylacetylene and ethyllithium:[3]
The capturing electrophile here is triisopropyl borate forming the boronic acid R–B(OH)2. The second step completing tamoxifen is a Suzuki reaction.
Silylmetalation
This reaction type can be extended to compounds of silicon bonded to a suitable metal in so-called silylmetalation as an extension to hydrosilylation. In one study[4] a zinc ate complex is formed from dimethylphenylsilyllithium, 2,2′-biphenol, zinc chloride and the Grignard reagent of tert-butylchloride which is capable of silylmetalation to styrene with 100% regioselectivity:
The intermediate complex can be captured with many electrophiles such as propargyl bromide (pictured, forming the allene), benzoyl chloride and allyl bromide.
References
- ↑ Shirakawa, Eiji; Yamagami, Takafumi; Kimura, Takahiro; Yamaguchi, Shigeru; Hayashi, Tamio (2005). "Arylmagnesiation of Alkynes Catalyzed Cooperatively by Iron and Copper Complexes". J. Am. Chem. Soc. (Communication). 127 (49): 17164–17165. doi:10.1021/ja0542136.
- ↑ In this reaction the Grignard reagent together with iron acetylacetonate and tributylphosphine first form an ill-defined aryliron intermediate and then by reaction with copper(I) chloride an intermediate cuprate.
- ↑ McKinley, Neola F.; O'Shea, Donal F. (2006). "Carbolithiation of Diphenylacetylene as a Stereoselective Route to (Z)-Tamoxifen and Related Tetrasubstituted Olefins". J. Org. Chem. (Note). 71 (25): 9552–9555. doi:10.1021/jo061949s.
- ↑ Nakamura, Shinji; Uchiyama, Masanobu (2007). "Regio- and Chemoselective Silylmetalation of Functionalized Terminal Alkenes". J. Am. Chem. Soc. (Communication). 129 (1): 28–29. doi:10.1021/ja066864n.