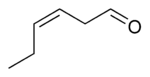
cis-3-Hexenal
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(3Z)-Hex-3-enal | |
| Other names
(Z)-Hex-3-enal cis-3-Hexenal Leaf aldehyde | |
| Identifiers | |
| 6789-80-6 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:23292 |
| ChemSpider | 559032 |
| ECHA InfoCard | 100.027.141 |
| PubChem | 643941 |
| UNII | 6V54TKA96C |
| |
| |
| Properties | |
| C6H10O | |
| Molar mass | 98.15 g·mol−1 |
| Density | 0.851 g/cm3 |
| Boiling point | 126 °C (259 °F; 399 K) |
| Related compounds | |
| Related alkenals |
Acrolein |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
cis-3-Hexenal, also known as (Z)-3-hexenal and leaf aldehyde, is colorless liquid and an aroma compound with an intense grassy-green odor of freshly cut green grass and leaves.[1][2] It is one of the major volatile compounds in ripe tomatoes. It is produced in small amounts by most plants and it acts as an attractant to many predatory insects. It is also a pheromone in many insect species.[3]
cis-3-Hexenal is an aldehyde. It is relatively unstable and isomerizes into the conjugated trans-2-hexenal. The related alcohol cis-3-hexen-1-ol is much more stable. It has a similar but weaker smell and is widely used in flavors and perfumes.
See also
- 1-Hexanol, another volatile organic compound, also considered responsible for the freshly mowed grass odor
References
- ↑ Molecule of the Month: Hexenal
- ↑ Hexenal / Chemistry World, Royal Society of Chemistry, 27 November 2013
- ↑ Pheromone database
This article is issued from Wikipedia - version of the 9/7/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.