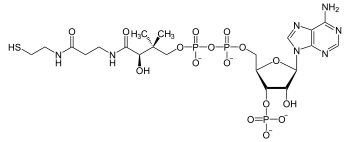
Coenzyme A
 | |
 | |
| Identifiers | |
|---|---|
| 85-61-0 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:15346 |
| ChEMBL | ChEMBL1213327 |
| ChemSpider | 6557 |
| DrugBank | DB01992 |
| ECHA InfoCard | 100.001.472 |
| KEGG | C00010 |
| MeSH | Coenzyme+A |
| PubChem | 6816 |
| UNII | SAA04E81UX |
| |
| |
| Properties | |
| C21H36N7O16P3S | |
| Molar mass | 767.535 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Coenzyme A (CoA, CoASH, or HSCoA) is a coenzyme, notable for its role in the synthesis and oxidation of fatty acids, and the oxidation of pyruvate in the citric acid cycle. All genomes sequenced to date encode enzymes that use coenzyme A as a substrate, and around 4% of cellular enzymes use it (or a thioester, such as acetyl-CoA) as a substrate. In humans, CoA biosynthesis requires cysteine, pantothenate, and adenosine triphosphate (ATP).[1]
Biosynthesis
In all living organisms, Coenzyme A is synthesized in a five-step process that requires four molecules of ATP, from pantothenate and cysteine:[2]
- Pantothenate (vitamin B5) is phosphorylated to 4'-phosphopantothenate by the enzyme pantothenate kinase (PanK; CoaA; CoaX)
- A cysteine is added to 4'-phosphopantothenate by the enzyme phosphopantothenoylcysteine synthetase (PPCS; CoaB) to form 4'-phospho-N-pantothenoylcysteine.
- PPC is decarboxylated to 4'-phosphopantetheine by phosphopantothenoylcysteine decarboxylase (PPC-DC; CoaC)
- 4'-phosphopantetheine is adenylylated to form dephospho-CoA by the enzyme phosphopantetheine adenylyl transferase (PPAT; CoaD)
- Finally, dephospho-CoA is phosphorylated to coenzyme A by the enzyme dephosphocoenzyme A kinase (DPCK; CoaE).
Enzyme nomenclature abbreviations in parentheses represent eukaryotic and prokaryotic enzymes respectively. In some plants and bacteria, including Escherichia coli, pantothenate can be synthesised de novo and is therefore not considered essential.
Discovery of structure
The structure of coenzyme A was identified in the early 1950s at the Lister Institute, London, together with other workers at Harvard Medical School and Massachusetts General Hospital.[3]
Function
Since coenzyme A is, in chemical terms, a thiol, it can react with carboxylic acids to form thioesters, thus functioning as an acyl group carrier. It assists in transferring fatty acids from the cytoplasm to mitochondria. A molecule of coenzyme A carrying an acetyl group is also referred to as acetyl-CoA. When it is not attached to an acyl group, it is usually referred to as 'CoASH' or 'HSCoA'.
Coenzyme A is also the source of the phosphopantetheine group that is added as a prosthetic group to proteins such as acyl carrier protein and formyltetrahydrofolate dehydrogenase.[4][5]
Use in biological research
Coenzyme A is available from various chemical suppliers as the free acid and or lithium or sodium salts. The free acid of coenzyme A is detectably unstable, with ~5% degradation observed after 6 months when stored at -20˚C,[6] and near complete degradation after 1 month at 37˚C. [7] The lithium and sodium salts of CoA are more stable, with negligible degradation noted over several months at various temperatures [8] Aqueous solutions of coenzyme A are unstable above pH 8, with 31% of activity lost after 24 hours at 25˚C and pH 8. CoA stock solutions are relatively stable when frozen at pH 2-6. The major route of CoA activity loss is likely the air oxidation of CoA to CoA disulfides. CoA mixed disulfides, such as CoA-S-S-glutathione, are commonly noted contaminants in commercial preparations of CoA.,[6] Free CoA can be regenerated from CoA disulfide and mixed CoA disulfides with reducing agents such as DTT or BME.
Non-exhaustive list of coenzyme A-activated acyl groups
- Acetyl-CoA
- fatty acyl-CoA (activated form of all fatty acids; only the CoA esters are substrates for important reactions such as mono-, di-, and triacylglycerol synthesis, carnitine palmitoyl transferase, and cholesterol esterification)
- Propionyl-CoA
- Butyryl-CoA
- Myristoyl-CoA
- Crotonyl-CoA
- Acetoacetyl-CoA
- Coumaroyl-CoA (used in flavonoid and stilbenoid biosynthesis)
- Benzoyl-CoA
- Phenylacetyl-CoA
- Acyl derived from dicarboxylic acids
- Malonyl-CoA
- Succinyl-CoA (used in heme biosynthesis)
- Hydroxymethylglutaryl-CoA (used in isoprenoid biosynthesis)
- Pimelyl-CoA (used in biotin biosynthesis)
Additional images
 Coenzyme A
Coenzyme A
References
- ↑ Matthew Daugherty; Boris Polanuyer; Michael Farrell; Michael Scholle; Athanasios Lykidis; Valérie de Crécy-Lagard; Andrei Osterman (2002). "Complete Reconstitution of the Human Coenzyme A Biosynthetic Pathway via Comparative Genomics". The Journal of Biological Chemistry. 277 (24): 21431–21439. doi:10.1074/jbc.M201708200. PMID 11923312.
- ↑ Leonardi R, Zhang YM, Rock CO, Jackowski S (2005). "Coenzyme A: back in action". Progress in Lipid Research. 44 (2-3): 125–153. doi:10.1016/j.plipres.2005.04.001. PMID 15893380.
- ↑ Baddiley, J.; Thain, E. M.; Novelli, G. D.; Lipmann, F. (1953). "Structure of Coenzyme A". Nature. 171 (4341): 76. doi:10.1038/171076a0.
- ↑ Elovson J, Vagelos PR (July 1968). "Acyl carrier protein. X. Acyl carrier protein synthetase". J. Biol. Chem. 243 (13): 3603–11. PMID 4872726.
- ↑ Strickland KC, Hoeferlin LA, Oleinik NV, Krupenko NI, Krupenko SA (January 2010). "Acyl carrier protein-specific 4'-phosphopantetheinyl transferase activates 10-formyltetrahydrofolate dehydrogenase". J. Biol. Chem. 285 (3): 1627–33. doi:10.1074/jbc.M109.080556. PMC 2804320
 . PMID 19933275.
. PMID 19933275. - 1 2 Dawson, R. M. C. (1989). Data for biochemical research. Oxford: Clarendon Press. p. 118-119. ISBN 0-19-855299-8.
- ↑ "Datasheet for free acid coenzyme A" (PDF). Oriental Yeast Co., LTD.
- ↑ "Datasheet for lithium salt coenzyme A" (PDF). Oriental Yeast Co., LTD.
Bibliography
- Nelson, David L.; Cox, Michael M. (2005). Lehninger: Principles of Biochemistry (4th ed.). New York: W.H. Freeman. ISBN 0-7167-4339-6.
| Wikimedia Commons has media related to Coenzyme A. |