Ethanol metabolism
Ethanol is metabolized through a very complex catabolic metabolic pathway.
Human metabolic physiology
Ethanol and evolution
Research suggest that animals evolved the ability to metabolize the alcohol present in fermented fruit to adapt to the changing climate 10 million years ago. Thanks to enzymes in their gut, and particularly one called ADH4, they can make use of the calories in alcohol.[1]
The average human digestive system produces approximately 3g of ethanol per day merely through fermentation of its contents.[2] Catabolic degradation of ethanol is thus essential to life, not only of humans, but of almost all living organisms. In fact, certain amino acid sequences in the enzymes used to oxidize ethanol are conserved all the way back to single cell bacteria.[3] Such a functionality is needed because all organisms actually produce alcohol in small amounts by several pathways, primarily along the fatty acid synthesis,[4] glycerolipid metabolism,[5] and bile acid biosynthesis pathways.[6] If the body had no mechanism for catabolizing the alcohols, they would build up in the body and become toxic. This could be an evolutionary rationale for alcohol catabolism also by sulfotransferase.
Physiologic structures
As is a basic organizing theme in biological systems, greater complexity of a body system, such as tissues and organs allows for greater specificity of function. This occurs for the processing of ethanol in the human body. All the enzymes needed to accomplish the oxidation reactions are confined to certain tissues. In particular, much higher concentration of such enzymes are found in the kidneys and in the liver,[7] making such organs the primary site for alcohol catabolism. Variations in genes influence alcohol metabolism and drinking behavior.[8]
Thermodynamic considerations
Energy thermodynamics
Energy calculations
The reaction from ethanol to carbon dioxide and water is a complex one that proceeds in three steps. Below, the Gibbs Free Energy of Formation for each step is shown with ΔGf values given in the CRC.[9]
Complete reaction: C2H6O(Ethanol)→C2H4O(Acetaldehyde)→C2H4O2(acetic Acid) →Acetyl-CoA→3H2O+2CO2.
ΔGf = Σ ΔGfp − ΔGfo
- Step One
Ethanol: −174.8 kJ/mol
Ethanal(Acetaldehyde): −127.6 kJ/mol
ΔGf1 = −127.6 + 174.8 = 47.2 kJ/mol(Endergonic)
ΣΔGf = 47.2 kJ/mol (Endergonic)
- Step Two
Ethanal: −127.6 kJ/mol
Acetic Acid: −389.9 kJ/mol
ΔGf2 = −389.9 + 127.6 = −262.3 kJ/mol (Exergonic)
ΣΔGf = = −262.3 + 47.2 = −215.1 kJ/mol (Exergonic)
- Step Three
- (Because the Gibbs energy is a state function, we can skip the Acetyl-CoA (step 3), for which thermodynamic values are not known).
Acetic Acid: −389.9 kJ/mol
3H2O+2CO2: −1 500.1 kJ/mol
ΔGf4 = −1 500 + 389.6 = −1 110.5 kJ/mol (Exergonic)
ΣΔGf = = −1 110.5 - 215.1 = −1 325.6 kJ/mol (Exergonic)
Discussion of calculations
If catabolism of alcohol goes all the way to completion, then, we have a very exothermic event yielding some 1 325 kJ/mol of energy. If the reaction stops part way through the metabolic pathways, which happens because acetic acid is excreted in the urine after drinking, then not nearly as much energy can be derived from alcohol, indeed, only 215.1 kJ/mol. At the very least, the theoretical limits on energy yield are determined to be -215.1 kJ/mol to -1 325.6 kJ/mol. It is also important to note that step 1 on this reaction is endothermic, requiring 47.2 kJ/mol of alcohol, or about 3 molecules of ATP (adenosine triphosphate) per molecule of ethanol.
Organic reaction scheme
Steps of the reaction
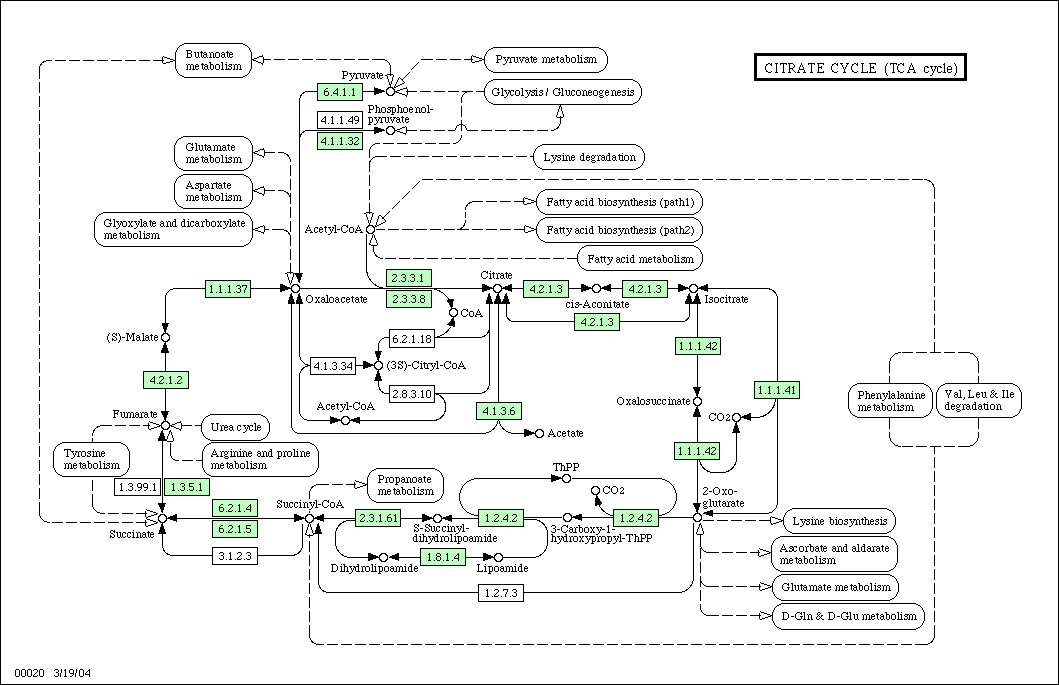
The first three steps of the reaction pathways lead from ethanol to acetaldehyde to acetic acid to acetyl-CoA. Once acetyl-CoA is formed, it is free to enter directly into the citric acid cycle.
Organic reactions
The reactions that transform ethanol into an aldehyde and then into a carboxylic acid are examples of oxidation reactions, which in organic chemistry, are typically characterized by the addition of oxygen onto a functional group.[10] The third reaction, the enzyme mediated formation of acetyl-CoA from acetic acid is an example of an enzymatic synthetase reaction where, through a complex intramolecular interaction a product molecule is formed from reactants.
Gene expression and ethanol metabolism
Ethanol to acetaldehyde in human adults
In human adults, ethanol is oxidized to acetaldehyde mainly via the hepatic enzyme alcohol dehydrogenase IB (class I), beta polypeptide (ADH1B, EC 1.1.1.1). The gene coding for this enzyme is located on chromosome 4, locus 4q21-q23. The enzyme encoded by this gene is a member of the alcohol dehydrogenase family. Members of this enzyme family metabolize a wide variety of substrates, including ethanol, retinol, other aliphatic alcohols, hydroxysteroids, and lipid peroxidation products. This encoded protein, consisting of several homo- and heterodimers of alpha, beta, and gamma subunits, exhibits high activity for ethanol oxidation and plays a major role in ethanol catabolism. Three genes encoding alpha, beta and gamma subunits are tandemly organized in a genomic segment as a gene cluster.[11]
Ethanol to acetaldehyde in human fetuses
In human embryos and fetuses, ethanol is not metabolized via this mechanism as ADH enzymes are not yet expressed to any significant quantity in human fetal liver (the induction of ADH only starts after birth, and requires years to reach adult levels).[12] Accordingly, the fetal liver cannot metabolize ethanol or other low molecular weight xenobiotiocs. In fetuses, ethanol is instead metabolized at much slower rates by different enzymes from the cytochrome P-450 superfamily (CYP), in particular by CYP2E1. The low fetal rate of ethanol clearance is responsible for the important observation that the fetal compartment retains high levels of ethanol long after ethanol has been cleared from the maternal circulation by the adult ADH activity in the maternal liver.[13] CYP2E1 expression and activity have been detected in various human fetal tissues after the onset of organogenesis (ca 50 days of gestation).[14] Exposure to ethanol is known to promote further induction of this enzyme in fetal and adult tissues. CYP2E1 is a major contributor to the so-called Microsomal Ethanol Oxidizing System (MEOS)[15] and its activity in fetal tissues is thought to contribute significantly to the toxicity of maternal ethanol consumption.[16][17] In presence of ethanol and oxygen, CYP2E1 is known to release superoxide radicals and induce the oxidation of polyunsaturated fatty acids to toxic aldehyde products like 4-hydroxynonenal (HNE).
Acetaldehyde to acetic acid
Acetaldehyde is a highly unstable compound and quickly forms free radical structures which are highly toxic if not quenched by antioxidants such as ascorbic acid (Vitamin C) and Vitamin B1 (thiamine). These free radicals can result in damage to embryonic neural crest cells and can lead to severe birth defects. Prolonged exposure of the kidney and liver to these compounds in chronic alcoholics can lead to severe damage.[18] The literature also suggests that these toxins may have a hand in causing some of the ill effects associated with hang-overs.
The enzyme associated with the chemical transformation from acetaldehyde to acetic acid is aldehyde dehydrogenase 2 family (ALDH2, EC 1.2.1.3). The gene coding for this enzyme is found on chromosome 12, locus q24.2.[19]
"This protein belongs to the aldehyde dehydrogenase family of proteins. Aldehyde dehydrogenase is the second enzyme of the major oxidative pathway of alcohol metabolism. Two major liver isoforms of aldehyde dehydrogenase, cytosolic and mitochondrial, can be distinguished by their electrophoretic mobilities, kinetic properties, and subcellular localizations. Most Caucasians have two major isozymes, while approximately 50% of East Asians have the cytosolic isozyme but not the mitochondrial isozyme. A remarkably higher frequency of acute alcohol intoxication among East Asians than among Caucasians could be related to the absence of a catalytically active form of the mitochondrial isozyme. The increased exposure to acetaldehyde in individuals with the catalytically inactive form may also confer greater susceptibility to many types of cancer. This gene encodes a mitochondrial isoform, which has a low Km for acetaldehydes, and is localized in mitochondrial matrix. Alternative splicing results in multiple transcript variants encoding distinct isoforms." [20]
Acetic acid to acetyl-CoA
Two enzymes are associated with the conversion of acetic acid to acetyl-CoA. The first is ACSS2 or acetyl CoA synthase-1 (EC 6.2.1.1), which is expressed by a gene located on chromosome 20, locus q11.22. "This gene encodes a nuclear-cytosolic enzyme that catalyzes the activation of acetate for use in lipid synthesis and protein acetylation reactions. The second enzyme is ACSS1 (acetyl CoA synthase-2) which is localized to mitochondria and is used for energy generation via the tricarboxylic acid cycle. The proteins act as monomers and produce acetyl-CoA from acetate in a reaction that requires ATP. Expression of ACSS2 is regulated by sterol regulatory element-binding proteins, transcription factors that activate genes required for the synthesis of cholesterol and unsaturated fatty acids. Two transcript variants encoding different isoforms have been found for this gene."[21]
 Gene 6.2.1.1 on Chromosome 20
Gene 6.2.1.1 on Chromosome 20
Acetyl-CoA to water and carbon dioxide
Once acetyl-CoA is formed, it enters the normal citric acid cycle.
References
- ↑ http://ww2.kqed.org/bayareabites/2014/12/03/our-ability-to-digest-alcohol-may-have-been-key-to-our-survival/
- ↑ ETHANOL, ACETALDEHYDE AND GASTROINTESTINAL FLORA Jyrki Tillonen ISBN 952-91-2603-4 PDF
- ↑ Comparison of Nucleotide Residues Between Humans and Bacteria
- ↑ Fatty Acid Synthesis
- ↑ Glycerolipid Metabolism
- ↑ Bile Acid Biosynthesis
- ↑ Polymorphism of alcohol-metabolizing genes affects drinking behavior and alcoholic liver disease in Japanese men
- ↑ "Genetic polymorphisms of alcohol metabolizing enzymes.". Pathol Biol (Paris). 49: 703–9. Nov 2001. PMID 11762132.
- ↑ CRC Handbook of Chemistry and Physics, 81st Edition, 2000
- ↑ J. McMurry, Organic Chemistry 6t ed. (United States: Thomson, 2004), 587–854.
- ↑ ADH1B at NIH
- ↑ Ernst van Faassen and Onni Niemelä, Biochemistry of prenatal alcohol exposure, NOVA Science Publishers, New York 2011.
- ↑ A. Nava-Ocampo et al, Reprod Toxicol 18 (2004) 613 - 617.
- ↑ M. Brzezinski et al, J. Pharmacol Exp Therap 289 (1999) 1648 - 1653.
- ↑ Charles Lieber, Drug Metab Rev 36 (2004) 511 - 529
- ↑ Ernst van Faassen and Onni Niemelä, Biochemistry of prenatal alcohol exposure, NOVA Science Publishers, New York 2011.
- ↑ Pregnancy and Alcohol Consumption, ed. J.D. Hoffmann, NOVA Science Publishers, New York 2011.
- ↑ http://ntp.niehs.nih.gov/ntp/roc/eleventh/profiles/s001acet.pdf
- ↑ NCBI sequence: locus NC_000012;43439 bp;DNA
- ↑ ALDH2 NIH
- ↑ ACSS2 NIH
Further reading
- Carrigana, MA, Uryasev O; et al. (2014). "Hominids adapted to metabolize ethanol long before human-directed fermentation.". Proc. Natl. Acad. Sci. USA. 112 (2): 599–608. doi:10.1073/pnas.1404167111. PMC 4299227
 . PMID 25453080.
. PMID 25453080.