Filbertone
 | |
| Names | |
|---|---|
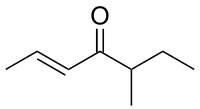
| IUPAC name
(2E)-5-Methyl-2-hepten-4-one | |
| Identifiers | |
| 102322-83-8 135910-94-0 (R) 122440-59-9 (S) | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 4515100 |
| ECHA InfoCard | 100.133.148 |
| PubChem | 5362588 |
| UNII | 8K2S58736F |
| |
| |
| Properties | |
| C8H14O | |
| Molar mass | 126.20 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Filbertone is the principal flavor compound of hazelnuts.[1] It is used in perfumery and is designated as generally recognized as safe (GRAS) for use in foods.[2]
Because filbertone is found in hazelnut oil, its presence can be used to detect the adulteration of olive oil with less expensive hazelnut oil.[3][4]
The natural compound is mixture of both enantiomers, and the composition can vary depending on the source.[5][6]
References
- ↑ Jauch, Johann; Schmalzing, Dieter; Schurig, Volker; Emberger, Roland; Hopp, Rudolf; Köpsel, Manfred; Silberzahn, Wilhelm; Werkhoff, Peter (1989). "Isolation, Synthesis, and Absolute Configuration of Filbertone - the Principal Flavor Component of the Hazelnut". Angewandte Chemie International Edition in English. 28 (8): 1022. doi:10.1002/anie.198910221.
- ↑ Zarbin, Paulo H.G.; Yonashiro, Massami; Perissini Jr, Waldir (1998). "An Alternative Route for the Synthesis of (E)-(+)-5(S)-Methylhept-2-en-4-one (Filbertone)". Journal of the Brazilian Chemical Society. 9 (6): 583. doi:10.1590/S0103-50531998000600011.
- ↑ Ruiz Del Castillo, María Luisa; Caja, María del Mar; Herraiz, Marta; Blanch, Gracia P. (1998). "Rapid Recognition of Olive Oil Adulterated with Hazelnut Oil by Direct Analysis of the Enantiomeric Composition of Filbertone". Journal of Agricultural and Food Chemistry. 46 (12): 5128. doi:10.1021/jf9807014.
- ↑ Flores, Gema; Ruiz Del Castillo, Maria Luisa; Herraiz, Marta; Blanch, Gracia Patricia (2006). "Study of the adulteration of olive oil with hazelnut oil by on-line coupled high performance liquid chromatographic and gas chromatographic analysis of filbertone". Food Chemistry. 97 (4): 742. doi:10.1016/j.foodchem.2005.06.008.
- ↑ Ruiz Del Castillo, M. L.; Gómez Caballero, E.; Blanch, G. P.; Herraiz, M. (2002). "Enantiomeric composition of filbertone in hazelnuts and hazelnut oils from different geographical origins". Journal of the American Oil Chemists' Society. 79 (6): 589. doi:10.1007/s11746-002-0527-1.
- ↑ Güntert, Matthias; Emberger, Roland; Hopp, Rudolf; Köpsel, Manfred; Silberzahn, Wilhelm; Werkhoff, Peter (1991). "Chirospecific analysis in flavor and essential oil chemistry Part A. Filbertone ? The character impact compound of hazel-nuts". Zeitschrift für Lebensmittel-Untersuchung und -Forschung. 192 (2): 108. doi:10.1007/BF01202621.
External links
- Filbertone, Molecule of the Month, University of Bristol
This article is issued from Wikipedia - version of the 8/3/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.