Humulone
-Humulone.svg.png) | |
 | |
| Names | |
|---|---|
| IUPAC name
(6S)-3,5,6-Trihydroxy-2-(3-methylbutanoyl)-4,6-bis(3-methylbut-2-en-1-yl)cyclohexa-2,4-dien-1-one[1] | |
| Other names
α-Lupulic acid; α-Bitter acid | |
| Identifiers | |
| 26472-41-3 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 391214 |
| ECHA InfoCard | 100.043.371 |
| PubChem | 442911 |
| UNII | KHB4767H3K |
| |
| |
| Properties | |
| C21H30O5 | |
| Molar mass | 362.47 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Humulone (α-lupulic acid), a vinylogous type of organic acid, is a bitter-tasting chemical compound found in the resin of mature hops (Humulus lupulus).[2] Humulone is a prevalent member of the class of compounds known as alpha acids, which collectively give hopped beer its characteristic bitter flavor.
Chemistry
In terms of structure, humulone is a phloroglucinol derivative with three isoprenoid side-chains. Two side-chains are prenyl groups and one is an isovaleryl group. The acidity of the ring enol moieties that give rise to its designation as an acid lie in their vinylogous relationship with the ring and side chain carbonyl functional groups.
Isohumulone
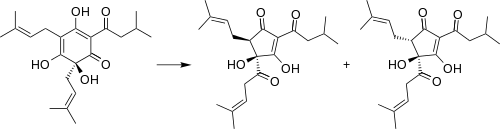
During the brewing process, humulone degrades to cis- and trans-isohumulone.[1] These “alpha acids” survive the boiling process, although numerous oxidized derivatives are produced.[3] The iso-alpha acids are significantly more soluble than humulone at the pH levels typically present in the brewing process.[4]
Laboratory synthesis
Humulone can be synthesized by the acylation of 1,2,3,5-benzenetetrol with isovaleryl chloride to give 2,3,4,6-tetrahydroxyisovalerophenone. This step is followed by prenylation with 1-bromo-3-methyl-2-butene to give humulone.[5]
 Synthesis of humulone from 1,2,3,5-tetrahydroxybenzene
Synthesis of humulone from 1,2,3,5-tetrahydroxybenzene
Biosynthesis
The biosynthesis of humulone in Humulus lupulus starts with an isovaleryl-CoA unit and 3 malonyl-CoA units catalyzed by phlorovalerophenone synthase. This conversion yields the benzenoid 3-methyl-1-(2,4,6-trihydroxyphenyl)butan-1-one. Dimethylallyl pyrophosphate is then obtained from the deoxyxylulose pathway, where prenylation of the benzenoid occurs, yielding humulone.[6]
- isovaleryl-CoA + 3 malonyl-CoA → 4 CoASH + 3 CO2 + 3-methyl-1-(2,4,6-trihydroxyphenyl)butan-1-one
- 3-methyl-1-(2,4,6-trihydroxyphenyl)butan-1-one + 2 DMAPP →C21H30O5
Biological properties
Studies of humulone have determined that it possesses a variety of biological activities in vitro including antioxidant[7][8] and cyclooxygenase-2 inhibitory activities.[9][10] Antimicrobial properties of humulone are antiviral[11] and antibacterial.[7][12][13][14]
References
- 1 2 Urban, Jan; Dahlberg, Clinton; Carroll, Brian; Kaminsky, Werner (2013). "Absolute Configuration of Beer′s Bitter Compounds". Angew. Chem. Int. Ed. 52 (5): 1553–1555. doi:10.1002/anie.201208450.
- ↑ De Keukeleire, J; Ooms, G; Heyerick, A; Roldan-Ruiz, I; Van Bockstaele, E; De Keukeleire, D (2003). "Formation and accumulation of alpha-acids, beta-acids, desmethylxanthohumol, and xanthohumol during flowering of hops (Humulus lupulus L.)". Journal of Agricultural and Food Chemistry. 51 (15): 4436–41. doi:10.1021/jf034263z. PMID 12848522.
- ↑ Blanco, C. A.; Rojas, A; Caballero, P. A.; Ronda, F; Gomez, M; Caballero, I., “A better control of beer properties by predicting acidity of hop iso-α-acids”, Trends in Food Science & Technology, Volume 17, Issue 7, July 2006, Pages 373-377, ISSN 0924-2244, doi:10.1016/j.tifs.2005.11.012.
- ↑ Esslinger, H. M. and Narziss, L. 2003. “Beer.” in- Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA, 2009 doi:10.1002/14356007.a03_421
- ↑ Obara; et al. (1989). "A Synthetic Route to (±)-humulone". Bull. Chem. Soc. Jpn. 62 (9): 3034–3035. doi:10.1246/bcsj.62.3034.
- ↑ M. Goese; K. Kammhuber; A. Bacher; M. H. Zenk; W. Eisenreich (1999). "Biosynthesis of bitter acids in hops. A (13)C-NMR and (2)H-NMR study on the building blocks of humulone". The Federation of European Biochemical Societies Journal. 263: 447–454.
- 1 2 Yamaguchi, N; Satoh-Yamaguchi, K; Ono, M (2009). "In vitro evaluation of antibacterial, anticollagenase, and antioxidant activities of hop components (Humulus lupulus) addressing acne vulgaris". Phytomedicine : international journal of phytotherapy and phytopharmacology. 16 (4): 369–76. doi:10.1016/j.phymed.2008.12.021. PMID 19201179.
- ↑ Tagashira, M; Watanabe, M; Uemitsu, N (1995). "Antioxidative activity of hop bitter acids and their analogues". Bioscience, Biotechnology, and Biochemistry. 59 (4): 740–2. doi:10.1271/bbb.59.740. PMID 7772843.
- ↑ Yamamoto, K; Wang, J; Yamamoto, S; Tobe, H (2000). "Suppression of cyclooxygenase-2 gene transcription by humulon of beer hop extract studied with reference to glucocorticoid". FEBS Letters. 465 (2–3): 103–6. doi:10.1016/S0014-5793(99)01727-5. PMID 10631313.
- ↑ Lee, JC; Kundu, JK; Hwang, DM; Na, HK; Surh, YJ (2007). "Humulone inhibits phorbol ester-induced COX-2 expression in mouse skin by blocking activation of NF-kappaB and AP-1: IkappaB kinase and c-Jun-N-terminal kinase as respective potential upstream targets". Carcinogenesis. 28 (7): 1491–8. doi:10.1093/carcin/bgm054. PMID 17372274.
- ↑ "Beer is good for you: study finds anti-virus powers". AFP. December 2012.
- ↑ Lewis, JC; Alderton, G; Carson, JF; Reynolds, DM; MacLay, WD (1949). "Lupulon and Humulon-Antibiotic Constituents of Hops". The Journal of Clinical Investigation. 28 (5 Pt 1): 916–9. doi:10.1172/JCI102178. PMC 438924
 . PMID 16695762.
. PMID 16695762. - ↑ Erdmann, WF (1951). "Phytoncides. I. Lupulone and humulone; their antibacterial action and their use in tuberculous infections". Die Pharmazie. 6 (9): 442–51. PMID 14920156.
- ↑ Dumas, ER; Michaud, AE; Bergeron, C; Lafrance, JL; Mortillo, S; Gafner, S (2009). "Deodorant effects of a supercritical hops extract: Antibacterial activity against Corynebacterium xerosis and Staphylococcus epidermidis and efficacy testing of a hops/zinc ricinoleate stick in humans through the sensory evaluation of axillary deodorancy". Journal of Cosmetic Dermatology. 8 (3): 197–204. doi:10.1111/j.1473-2165.2009.00449.x. PMID 19735518.