Indigo carmine
 | |
| Names | |
|---|---|
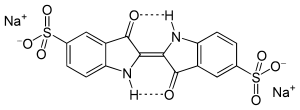
| IUPAC name
3,3′-dioxo-2,2′-bisindolyden-5,5′-disulfonic acid disodium salt | |
| Other names
indigotine 5,5′-indigodisulfonic acid sodium salt Brilliant Indigo4 G C.I. Acid Blue 74 C.I. 73015 Food Blue 1 C Blue 2 Sicovit Indigotin 85E 132. | |
| Identifiers | |
| 860-22-0 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:31695 |
| ChemSpider | 4447431 |
| ECHA InfoCard | 100.011.572 |
| E number | E132 (colours) |
| PubChem | 5284351 |
| UNII | D3741U8K7L |
| |
| |
| Properties | |
| C16H8N2Na2O8S2 | |
| Molar mass | 466.36 g/mol |
| Appearance | purple solid |
| Melting point | >300 °C (572 °F) |
| 10 g/L (25 °C (77 °F)) | |
| Hazards | |
| GHS pictograms |  [1] [1] |
| GHS signal word | Warning |
| H302[1] | |
| R-phrases | R22 |
| S-phrases | S22 S24/25 |
| NFPA 704 | |
| Pharmacology | |
| V04CH02 (WHO) | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Indigo carmine, or 5,5′-indigodisulfonic acid sodium salt, is an organic salt derived from indigo by sulfonation, which renders the compound soluble in water. It is approved for use as a food colorant in the U.S and E.U.,[2][3] It has the E number E132. It is also a pH indicator.
Uses
| Indigo Carmine (pH indicator) | ||
| below pH 11.4 | above pH 13.0 | |
| 11.4 | ⇌ | 13.0 |
Indigo carmine in a 0.2% aqueous solution is blue at pH 11.4 and yellow at 13.0. Indigo carmine is also a redox indicator, turning yellow upon reduction. Another use is as a dissolved ozone indicator[4] through the conversion to isatin-5-sulfonic acid.[4] This reaction has been shown not to be specific to ozone, however: it also detects superoxide, an important distinction in cell physiology.[5] It is also used as a dye in the manufacturing of capsules.
In obstetric surgery, indigo carmine solutions are sometimes employed to detect amniotic fluid leaks. In urologic surgery, intravenous injection of indigo carmine is often used to highlight portions of the urinary tract. The dye is filtered rapidly by the kidneys from the blood, and colors the urine blue. This enables structures of the urinary tract to be seen in the surgical field, and demonstrate if there is a leak. However, the dye can cause a potentially dangerous increase in blood pressure in some cases.[6]
See also
References
- 1 2 "Indigo carmine". Sigma Aldrich. Retrieved 10 March 2016.
- ↑ Summary of Color Additives for Use in United States in Foods, Drugs, Cosmetics, and Medical Devices, United States Food and Drug Administration
- ↑ Current EU approved additives and their E Numbers, Food Standards Agency, 26 November 2010
- 1 2 Takeuchi K, Ibusuki T (March 1989). "Quantitative determination of aqueous-phase ozone by chemiluminescence using indigo-5,5'-disulfonate". Anal. Chem. 61 (6): 619–23. doi:10.1021/ac00181a025. PMID 2729594.
- ↑ Kettle AJ, Clark BM, Winterbourn CC (April 2004). "Superoxide converts indigo carmine to isatin sulfonic acid: implications for the hypothesis that neutrophils produce ozone". J. Biol. Chem. 279 (18): 18521–5. doi:10.1074/jbc.M400334200. PMID 14978029.
- ↑ Craik, Johnathan Donaldson (January–February 2009). "The Safety of Intravenous Indigo Carmine to Assess Ureteric Patency During Transvaginal Uterosacral Suspension of the Vaginal Vault". Journal of Pelvic Medicine & Surgery. 15 (1): 11–15. doi:10.1097/SPV.0b013e3181986ace. Retrieved 18 October 2012.
- http://physchem.ox.ac.uk/MSDS/IN/indigo_carmine.html
- http://hazard.com/msds/mf/baker/baker/files/i1440.htm
