Interleukin 15
| View/Edit Human | View/Edit Mouse |
Interleukin 15 (IL-15) is a cytokine with structural similarity to IL-2. Like IL-2, IL-15 binds to and signals through a complex composed of IL-2/IL-15 receptor beta chain (CD122) and the common gamma chain (gamma-C, CD132). IL-15 is secreted by mononuclear phagocytes (and some other cells) following infection by virus(es). This cytokine induces cell proliferation of natural killer cells; cells of the innate immune system whose principal role is to kill virally infected cells.
Expression
Interleukin - 15 (IL-15) was discovered in 1994 by two different laboratories, and characterized as T cell growth factor.[3] Together with interleukin 2 (IL-2), interleukin 4 (IL-4), interleukin 7 (IL-7), interleukin 9 (IL-9), granulocyte colony-stimulating factor (G-CSF), and granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-15 belongs to the four α-helix bundle family of cytokine.[3][4]
IL-15 is constitutively expressed by a large number of cell types and tissues, including monocytes, macrophages, dendritic cells (DC), keratinocytes, fibroblasts and nerve cells.[5] As a pleiotropic cytokine, it plays an important role in innate and adaptive immunity.[6]
Gene
IL -15 is 14-15 kDa glycoprotein encoded by the 34 kb region 4q31 of chromosome 4, and by the central region of chromosome 8 in mice.[7] The human IL-15 gene comprises nine exons (1 - 8 and 4A) and eight introns, four of which (exons 5 through 8) code for the mature protein (Figure 1).[3]

Two alternatively spliced transcript variants of this gene encoding the same protein have been reported.[8] The originally identified isoform, with long signal peptide of 48 amino acids (IL-15 LSP) consisted of a 316 bp 5’-untranslated region (UTR), 486 bp coding sequence and the C-terminus 400 bp 3’-UTR region. The other isoform (IL-15 SSP) has a short signal peptide of 21 amino acids encoded by exons 4A and 5.[3] Both isoforms shared 11 amino acids between signal sequences of the N-terminus.[9] Although both isoforms produce the same mature protein, they differ in their cellular trafficking.[3] IL-15 LSP isoform was identified in Golgi apparatus [GC], early endosomes and in the endoplasmic reticulum (ER). It exists in two forms, secreted and membrane-bound particularly on dendritic cells. On the other hand, IL-15 SSP isoform is not secreted and it appears to be restricted to the cytoplasm and nucleus where plays an important role in the regulation of cell cycle.[3]
It has been demonstrated that two isoforms of IL-15 mRNA are generated by alternatively splicing in mice. The isoform which had an alternative exon 5 containing another 3’ splicing site, exhibited a high translational efficiency, and the product lack hydrophobic domains in the signal sequence of the N-terminus. This suggests that the protein derived from this isoform is located intracellulary. The other isoform with normal exon 5, which is generated by integral splicing of the alternative exon 5, may be released extracellulary.[10]
Although IL-15 mRNA can be found in many cells and tissues including mast cells, cancer cells or fibroblasts, this cytokine is produce as a mature protein mainly by dendritic cells, monocytes and macrophages. This discrepancy between the wide appearance of IL-15 mRNA and limited production of protein might be explained by the presence of the twelve in humans and five in mice upstream initiating codons, which can repress translation of IL-15 mRNA. Translational inactive mRNA is stored within the cell and can be induced upon specific signal.[11] Expression of IL-15 can be stimulated by cytokine such as GM-CSF, double-strand mRNA, unmethylated CpG oligonucleotides, lipopolysaccharide (LPS) through Toll-like receptors (TLR), interferon gamma (IFN-γ) or after infection of monocytes herpes virus, Mycobacterium tuberculosis and Candida albicans (Figure 2).[12]

Signaling



The prevailing mechanism of IL-15 action seems to be juxtacrine signaling or also determined as cell-to-cell contact. It also includes intracrine and reverse signaling. IL-15 was initially characterized as a soluble molecule. Later it was shown that IL-15 also exists as a membrane-bound form which represents the major form of IL-15 protein. In membrane-bound form it could be bound directly to cellular membrane or presented by IL-15Rα receptor.[11]
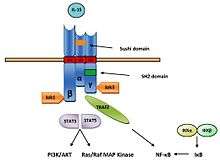
The main mechanism of IL-15 signaling is trans-presentation which is mediated by membrane-bound complex IL-15/IL-15Rα (Figure 3).[13] IL-15 bind to IL-15Rα receptor alone with affinity (Ka = 1.1011/M). It can also bind to IL-15Rβγc signaling complex with lower affinity (Ka = 1.109/M) (Figure 4).[6]
Signaling pathway of IL-15 begins with binding to IL-15Rα receptor, with subsequent presentation to surrounding cells bearing IL-15Rβγc complex on their cell surface. Upon binding IL-15β subunit activates Janus kinase 1 (Jak1) and γc subunit Janus kinase 3 (Jak3), which leads to phosphorylation and activation of signal transducer and activator of transcription 3 (STAT3) and STAT5.[14] Due to sharing of receptor subunits between IL-2 and IL-15, both of these cytokines have similar downstream effects including the induction of B-cell lymphoma (Bcl-2), MAP (mitogen-activated protein kinase) kinase pathway and the phosphorylation of Lck (lymphocyte-activated protein tyrosine kinase) and Syk (spleen tyrosine kinase) kinases, which leads to cell proliferation and maturation (Figure 5).[6][15]
In mast cells, the IL-15R signaling pathway has been found to include Jak2 and STAT5 instead Jak1/3 and STAT3/5. Phosphorylation STATs form transcription factors and activate transcription of appropriate genes. The β chain of IL-15R recruits and also activates protein tyrosine kinases of the Src family including Lck, Fyn and Lyn kinase. It also activates phosphatidylinositol 3-kinase (PI3K) and AKT signaling pathway and induce expression of transcription factors including c-Fos, c-Jun, c-Myc and NF-κB.[11]
IL-15 is also able to bind to the 15Rβγc signaling complex with intermediate affinity without requirement for IL-15Rα rreceptor. Upon binding IL-15 to signaling complex activates kinases of the Src family including Lck and Fyn, which subsequently activates PI3K and MAPK signaling pathway.[16] The second mechanism of IL-15 action is cis-presentation, when il-15 is presented by IL-15Rα to 15Rβγc signaling complex on the same cell. This mechanism is mediated by the C-terminus flexibility which is mediated by 32 amino acids linker and/or 74 amino acids long PT region (Figure 6).[13]
Function
Interleukin 15 (IL-15) regulates T and natural killer (NK) cell activation and proliferation. Survival signals that maintain memory T cells in the absence of antigen are provided by IL-15. This cytokine is also implicated in NK cell development. In rodent lymphocytes, IL-15 prevents apoptosis by inducing an apoptosis inhibitor, BCL2L1/BCL-x(L).[8] In humans with celiac disease IL-15 similarly suppresses apoptosis in T-lymphocytes by inducing Bcl-2 and/or Bcl-xL.[17]
A hematopoietin receptor, the IL-15 receptor, that binds IL-15 propagates its function. Some subunits of the IL-15 receptor are shared in common with the receptor for a structurally related cytokine called interleukin 2 (IL-2) allowing both cytokines to compete for and negatively regulate each other's activity. CD8+ memory T cell number is controlled by a balance between IL-15 and IL-2. When IL-15 binds its receptor, JAK kinase, STAT3, STAT5, and STAT6 transcription factors are activated to elicit downstream signaling events.
As a myokine, interleukin-15 appears to play a significant role in the reduction of visceral (intra-abdominal or interstitial) fat.[18]
Disease
Epstein-Barr virus
In humans with history of acute infectious mononucleosis (the syndrome associated with primary Epstein-Barr virus infection), IL-15R expressing lymphocytes are not detected—even 14 years after infection.[19]
Celiac disease
There have been recent studies suggesting that suppression of IL-15 may be a potential treatment for celiac disease and even presents the possibility of preventing its development. In one study with mice blocking IL-15 with an antibody led to the reversal of autoimmune intestinal damage.[20] In another study mice used were able to eat gluten without developing symptoms.[21]
Immunotherapy
Metastatic cancer
IL-15 has been shown to enhance the anti-tumor immunity of CD8+ T cells in pre-clinical models.[22][23] A phase I clinical trial to evaluate the safety, dosing, and anti-tumor efficacy of IL-15 in patients with metastatic melanoma and renal cell carcinoma (kidney cancer) has begun to enroll patients at the National Institutes of Health.[24]
Derivatives
ALT-803
A combined IL-15N72D mutant and the soluble domain of IL-15Rα, known as ALT-803, As of 2014 INDs have been submitted for clinical trials for 4 indications : metastatic melanoma, relapse of hematological malignancies after allogeneic stem cell transplantation, refractory multiple myeloma, and BCG-naïve non-muscle invasive bladder cancer in combination with BCG.[25] In 2016 it started a clinical trial for advanced or metastatic non-small cell lung cancer (NSCLC).[26]
References
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- 1 2 3 4 5 6 Steel JC, Waldmann TA, Morris JC (January 2012). "Interleukin-15 biology and its therapeutic implications in cancer". Trends Pharmacol. Sci. 33 (1): 35–41. doi:10.1016/j.tips.2011.09.004. PMC 3327885
 . PMID 22032984.
. PMID 22032984. - ↑ Di Sabatino A, Calarota SA, Vidali F, Macdonald TT, Corazza GR (February 2011). "Role of IL-15 in immune-mediated and infectious diseases". Cytokine Growth Factor Rev. 22 (1): 19–33. doi:10.1016/j.cytogfr.2010.09.003. PMID 21074481.
- ↑ Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn MA, Ahdieh M (May 1994). "Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor". Science. 264 (5161): 965–8. doi:10.1126/science.8178155. PMID 8178155.
- 1 2 3 Lodolce JP, Burkett PR, Koka RM, Boone DL, Ma A (December 2002). "Regulation of lymphoid homeostasis by interleukin-15". Cytokine Growth Factor Rev. 13 (6): 429–39. doi:10.1016/S1359-6101(02)00029-1. PMID 12401478.
- ↑ Waldmann TA, Tagaya Y (1999). "The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens". Annu. Rev. Immunol. 17: 19–49. doi:10.1146/annurev.immunol.17.1.19. PMID 10358752.
- 1 2 "Entrez Gene: IL15 interleukin 15".
- ↑ Tagaya Y, Kurys G, Thies TA, Losi JM, Azimi N, Hanover JA, Bamford RN, Waldmann TA (December 1997). "Generation of secretable and nonsecretable interleukin 15 isoforms through alternate usage of signal peptides". Proc. Natl. Acad. Sci. U.S.A. 94 (26): 14444–9. doi:10.1073/pnas.94.26.14444. PMC 25016
 . PMID 9405632.
. PMID 9405632. - ↑ Nishimura H, Yajima T, Naiki Y, Tsunobuchi H, Umemura M, Itano K, Matsuguchi T, Suzuki M, Ohashi PS, Yoshikai Y (January 2000). "Differential roles of interleukin 15 mRNA isoforms generated by alternative splicing in immune responses in vivo". J. Exp. Med. 191 (1): 157–70. doi:10.1084/jem.191.1.157. PMC 2195806
 . PMID 10620614.
. PMID 10620614. - 1 2 3 Jakobisiak M, Golab J, Lasek W (April 2011). "Interleukin 15 as a promising candidate for tumor immunotherapy". Cytokine Growth Factor Rev. 22 (2): 99–108. doi:10.1016/j.cytogfr.2011.04.001. PMID 21531164.
- ↑ Bamford RN, DeFilippis AP, Azimi N, Kurys G, Waldmann TA (May 1998). "The 5' untranslated region, signal peptide, and the coding sequence of the carboxyl terminus of IL-15 participate in its multifaceted translational control". J. Immunol. 160 (9): 4418–26. PMID 9574546.
- 1 2 Olsen SK, Ota N, Kishishita S, Kukimoto-Niino M, Murayama K, Uchiyama H, Toyama M, Terada T, Shirouzu M, Kanagawa O, Yokoyama S (December 2007). "Crystal Structure of the interleukin-15.interleukin-15 receptor alpha complex: insights into trans and cis presentation". J. Biol. Chem. 282 (51): 37191–204. doi:10.1074/jbc.M706150200. PMID 17947230.
- ↑ Okada S, Han S, Patel ES, Yang LJ, Chang LJ. "STAT3 signaling contributes to the high effector activities of interleukin-15-derived dendritic cells". Immunology and Cell Biology. 93 (5): 461–71. doi:10.1038/icb.2014.103. PMID 25582338.
- ↑ Schluns KS, Stoklasek T, Lefrançois L (August 2005). "The roles of interleukin-15 receptor alpha: trans-presentation, receptor component, or both?". Int. J. Biochem. Cell Biol. 37 (8): 1567–71. doi:10.1016/j.biocel.2005.02.017. PMID 15896666.
- ↑ Perera PY, Lichy JH, Waldmann TA, Perera LP (March 2012). "The role of interleukin-15 in inflammation and immune responses to infection: implications for its therapeutic use". Microbes Infect. 14 (3): 247–61. doi:10.1016/j.micinf.2011.10.006. PMC 3270128
 . PMID 22064066.
. PMID 22064066. - ↑ Malamut G, El Machhour R, Montcuquet N, Martin-Lannerée S, Dusanter-Fourt I, Verkarre V, Mention JJ, Rahmi G, Kiyono H, Butz EA, Brousse N, Cellier C, Cerf-Bensussan N, Meresse B (June 2010). "IL-15 triggers an antiapoptotic pathway in human intraepithelial lymphocytes that is a potential new target in celiac disease–associated inflammation and lymphomagenesis". J. Clin. Invest. 120 (6): 2131–43. doi:10.1172/JCI41344. PMC 2877946
 . PMID 20440074.
. PMID 20440074. - ↑ Pedersen, Bente Klarlund. "Muscles and their myokines." The Journal of Experimental Biology 214, 337-346. © 2011. Published by The Company of Biologists Ltd. doi:10.1242/jeb.048074
- ↑ Sauce D, Larsen M, Curnow SJ, Leese AM, Moss PA, Hislop AD, Salmon M, Rickinson AB (July 2006). "EBV-associated mononucleosis leads to long-term global deficit in T-cell responsiveness to IL-15". Blood. 108 (1): 11–8. doi:10.1182/blood-2006-01-0144. PMID 16543467.
- ↑ DePaolo RW, Abadie V, Tang F, Fehlner-Peach H, Hall JA, Wang W, Marietta EV, Kasarda DD, Waldmann TA, Murray JA, Semrad C, Kupfer SS, Belkaid Y, Guandalini S, Jabri B (March 2011). "Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens". Nature. 471 (7337): 220–4. doi:10.1038/nature09849. PMC 3076739
 . PMID 21307853. Lay summary – WebMD Health News.
. PMID 21307853. Lay summary – WebMD Health News. - ↑ Yokoyama S, Watanabe N, Sato N, Perera PY, Filkoski L, Tanaka T, Miyasaka M, Waldmann TA, Hiroi T, Perera LP (September 2009). "Antibody-mediated blockade of IL-15 reverses the autoimmune intestinal damage in transgenic mice that overexpress IL-15 in enterocytes". Proc. Natl. Acad. Sci. U.S.A. 106 (37): 15849–54. doi:10.1073/pnas.0908834106. PMC 2736142
 . PMID 19805228.
. PMID 19805228. - ↑ Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, Grewal N, Spiess PJ, Antony PA, Palmer DC, Tagaya Y, Rosenberg SA, Waldmann TA, Restifo NP (February 2004). "IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T Cells". Proc. Natl. Acad. Sci. U.S.A. 101 (7): 1969–74. doi:10.1073/pnas.0307298101. PMC 357036
 . PMID 14762166.
. PMID 14762166. - ↑ Teague RM, Sather BD, Sacks JA, Huang MZ, Dossett ML, Morimoto J, Tan X, Sutton SE, Cooke MP, Ohlén C, Greenberg PD (March 2006). "Interleukin-15 rescues tolerant CD8+ T cells for use in adoptive immunotherapy of established tumors". Nat. Med. 12 (3): 335–41. doi:10.1038/nm1359. PMID 16474399.
- ↑ "A Phase I Study of Intravenous Recombinant Human IL-15 in Adults With Refractory Metastatic Malignant Melanoma and Metastatic Renal Cell Cancer". ClinicalTrials.gov.
- ↑ ALT-803 Fact Sheet
- ↑ ALT-803 and Nivolumab Trial Launches for NSCLC. Jan 2016
Further reading
- Maślińska D (2001). "The cytokine network and interleukin-15 (IL-15) in brain development". Folia Neuropathologica. 39 (2): 43–7. PMID 11680634.
- Liew FY, McInnes IB (2002). "Role of interleukin 15 and interleukin 18 in inflammatory response". Ann. Rheum. Dis. 61 Suppl 2 (Suppl 2): ii100–2. doi:10.1136/ard.61.suppl_2.ii100. PMC 1766710
 . PMID 12379638.
. PMID 12379638. - Lodolce JP, Burkett PR, Koka RM, et al. (2003). "Regulation of lymphoid homeostasis by interleukin-15". Cytokine Growth Factor Rev. 13 (6): 429–39. doi:10.1016/S1359-6101(02)00029-1. PMID 12401478.
- Mattei Fabrizio; Schiavoni G.; Belardelli F.; Tough D.F. (2001). "IL-15 is expressed by dendritic cells in response to Type I IFN, Double-stranded RNA, or Lipopolysaccharide and promotes dendritic cell activation". J. Immunol. 167 (3): 1179–87. doi:10.4049/jimmunol.167.3.1179. PMID 11466332.