Bulk modulus
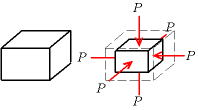
The bulk modulus ( or ) of a substance is a measure of how compressible that substance is. It is defined as the ratio of the infinitesimal pressure increase to the resulting relative decrease of the volume.[1]
Definition
The bulk modulus can be formally defined by the equation
where is pressure, is volume, and denotes the derivative of pressure with respect to volume. Equivalently
where ρ is density and dP/dρ denotes the derivative of pressure with respect to density (i.e. pressure rate of change with volume). The inverse of the bulk modulus gives a substance's compressibility.
Other moduli describe the material's response (strain) to other kinds of stress: the shear modulus describes the response to shear, and Young's modulus describes the response to linear stress. For a fluid, only the bulk modulus is meaningful. For an anisotropic solid such as wood or paper, these three moduli do not contain enough information to describe its behaviour, and one must use the full generalized Hooke's law.
Thermodynamic relation
Strictly speaking, the bulk modulus is a thermodynamic quantity, and in order to specify a bulk modulus it is necessary to specify how the temperature varies during compression: constant-temperature (isothermal ), constant-entropy (isentropic ), and other variations are possible. Such distinctions are especially relevant for gases.
For an ideal gas, the isentropic bulk modulus is given by
and the isothermal bulk modulus is given by
where
- γ is the heat capacity ratio
- p is the pressure.
When the gas is not ideal, these equations give only an approximation of the bulk modulus. In a fluid, the bulk modulus K and the density ρ determine the speed of sound c (pressure waves), according to the Newton-Laplace formula
In solids, and have very similar values. Solids can also sustain transverse waves: for these materials one additional elastic modulus, for example the shear modulus, is needed to determine wave speeds.
Measurement
It is possible to measure the bulk modulus using powder diffraction under applied pressure. It is a property of a fluid which shows its ability to change its volume under its pressure.
Selected values
| Material | Bulk modulus in GPa | Bulk modulus in psi |
|---|---|---|
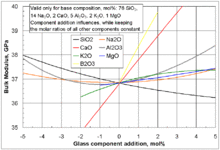
| Glass (see also diagram below table) | 35 to 55 | 5.8×106 |
| Steel | 160 | 23.2×106 |
| Diamond (at 4K) [2] | 443 | 64×106 |
A material with a bulk modulus of 35 GPa loses one percent of its volume when subjected to an external pressure of 0.35 GPa (~3500 bar).
| Water | 2.2×109 Pa (value increases at higher pressures) |
| Methanol | 8.23×108 Pa (at 20 °C and 1 Atm) |
| Air | 1.42×105 Pa (adiabatic bulk modulus) |
| Air | 1.01×105 Pa (constant temperature bulk modulus) |
| Solid helium | 5×107 Pa (approximate) |
References
- ↑ "Bulk Elastic Properties". hyperphysics. Georgia State University.
- ↑ Page 52 of "Introduction to Solid State Physics, 8th edition" by Charles Kittel, 2005, ISBN 0-471-41526-X
- ↑ Fluegel, Alexander. "Bulk modulus calculation of glasses". glassproperties.com.
Further reading
- De Jong, Maarten; Chen, Wei (2015). "Charting the complete elastic properties of inorganic crystalline compounds". Scientific Data. 2: 150009. doi:10.1038/sdata.2015.9.
| Conversion formulas | |||||||
|---|---|---|---|---|---|---|---|
| Homogeneous isotropic linear elastic materials have their elastic properties uniquely determined by any two moduli among these; thus, given any two, any other of the elastic moduli can be calculated according to these formulas. | |||||||
| Notes | |||||||
| | |||||||
| Cannot be used when | |||||||