Graphite intercalation compound
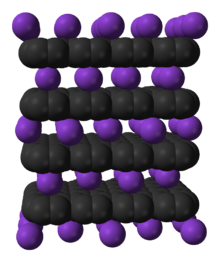
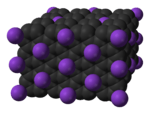
Graphite intercalation compounds (GICs) are complex materials having a formula CXm where the ion Xn+ or Xn− is inserted (intercalated) between the oppositely charged carbon layers. Typically m is much less than 1.[1][2] These materials are deeply colored solids that exhibit a range of electrical and redox properties of potential applications.
Preparation and structure
These materials are prepared by treating graphite with a strong oxidant or a strong reducing agent:
- C + m X → CXm
The reaction is reversible.
The host (graphite) and the guest X interact by charge transfer. A analogous process is the basis of commercial lithium-ion batteries.
In a graphite intercalation compound not every layer is necessarily occupied by guests. In so-called stage 1 compounds, graphite layers and intercalated layers alternate and in stage 2 compounds, two graphite layers with no guest material in between alternate with an intercalated layer. The actual composition may vary and therefore these compounds are an example of non-stoichiometric compounds. It is customary to specify the composition together with the stage. The layers are pushed apart upon incorporation of the guest ions.
Examples
Alkali and alkaline earth derivatives

One of the best studied graphite intercalation compounds, KC8, is prepared by melting potassium over graphite powder. The potassium is absorbed into the graphite and the material changes color from black to bronze.[3] The resulting solid is pyrophoric.[4] The composition is explained by assuming that the potassium to potassium distance is twice the distance between hexagons in the carbon framework. The bond between anionic graphite layers and potassium cations is ionic. The electrical conductivity of the material is greater than that of α-graphite.[4][5] KC8 is a superconductor with a very low critical temperature Tc = 0.14 K.[6] Heating KC8 leads to the formation of a series of decomposition products as the K atoms are eliminated:
- 3 KC8 → KC24 + 2 K
Via the intermediates KC24 (blue in color),[3] KC36, KC48, ultimately the compound KC60 results.
The stoichiometry MC8 is observed for M = K, Rb and Cs. For smaller ions M = Li+, Sr2+, Ba2+, Eu2+, Yb3+, and Ca2+, the limiting stoichiometry is MC6.[6] Calcium graphite CaC
6 is obtained by immersing highly oriented pyrolytic graphite in liquid Li–Ca alloy for 10 days at 350 °C. The crystal structure of CaC
6 belongs to the R3m space group. The graphite interlayer distance increases upon Ca intercalation from 3.35 to 4.524 Å, and the carbon-carbon distance increases from 1.42 to 1.444 Å.
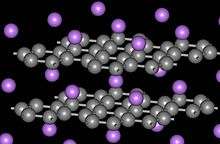
6
With barium and ammonia, the cations are solvated, giving the stoichiometry (Ba(NH3)2.5C10.9(stage 1)) or those with caesium, hydrogen and potassium (CsC8·K2H4/3C8(stage 1)).
Interestingly, different from other alkali metals, the amount of Na intercalation is very small. Quantum-mechanical calculations show that this originate from a quite general phenomenon: among the alkali and alkaline earth metals, Na and Mg generally have the weakest chemical binding to a given substrate, compared with the other elements in the same group of the periodic table.[7] The phenomenon arises from the competition between trends in the ionization energy and the ion–substrate coupling, down the columns of the periodic table.[7] However, considerable Na intercalation into graphite can occur in cases when the ion is wrapped in a solvent shell through the process of co-intercalation. Na ions can intercalate into graphite when using certain glyme based electrolytes.[8] Co-intercalation using glyme electrolytes can also facilitate faster potassium ion intercalation into graphite.[9]
Graphite bisulfate, perchlorate, hexafluoroarsenate: oxidized carbons
The intercalation compounds graphite bisulfate and graphite perchlorate can be prepared by treating graphite with strong oxidizing agents in the presence of strong acids. In contrast to the potassium and calcium graphites, the carbon layers are oxidized in this process: 48 C + 0.25 O2 + 3 H2SO4 → [C24]+[HSO4]−·2H2SO4 + 0.5 H2O
In graphite perchlorate, planar layers of carbon atoms are 794 picometers apart, separated by ClO4− ions. Cathodic reduction of graphite perchlorate is analogous to heating KC8, which leads to a sequential elimination of HClO4.
Both graphite bisulfate and graphite perchlorate are better conductors as compared to graphite, as predicted by using a positive-hole mechanism.[4] Reaction of graphite with [O2]+[AsF6]− affords the salt [C8]+[AsF6]−.[4]
Metal halide derivatives
A number of metal halides intercalate into graphite. The chloride derivatives have been most extensively studied. Examples include MCl2 (M = Zn, Ni, Cu, Mn), MCl3 (M = Al, Fe, Ga), MCl4 (M = Zr, Pt), etc.[1] The materials consists of layers of close-packed metal halide layers between sheets of carbon. The derivative C~8FeCl3 exhibits spin glass behavior.[10] It proved to be a particularly fertile system on which to study phase transitions. A stage n magnetic GIC has n graphite layers separating successive magnetic layers. As the stage number increases the interaction between spins in successive magnetic layers becomes weaker and 2D magnetic behaviour may arise.
Halogen- and oxide-graphite compounds
Chlorine and bromine reversibly intercalate into graphite. Iodine does not. Fluorine reacts irreversibly. In the case of bromine, the following stoichiometries are known: CnBr for n = 8, 12, 14, 16, 20, and 28.
Because it forms irreversibly, carbon monofluoride is often not classified as an intercalation compound. It has the formula (CF)x. It is prepared by reaction of gaseous fluorine with graphitic carbon at 215–230 °C. The color is greyish, white, or yellow. The bond between the carbon and fluorine atoms is covalent. Tetracarbon monofluoride (C4F) is prepared by treating graphite with a mixture of fluorine and hydrogen fluoride at room temperature. The compound has a blackish-blue color. Carbon monofluoride is not electrically conductive. It has been studied as a cathode material in one type of primary (non-rechargeable) lithium batteries.
Graphite oxide is an unstable yellow solid.
Properties and applications
Graphite intercalation compounds have fascinated materials scientists for many years owing to their diverse electronic and electrical properties.
Superconductivity
Among the superconducting graphite intercalation compounds, CaC
6 exhibits the highest critical temperature Tc = 11.5 K, which further increases under applied pressure (15.1 K at 8 GPa).[6] Superconductivity in these compounds is thought to be related to the role of an interlayer state, a free electron like band lying roughly 2 eV (0.32 aJ) above the Fermi level; superconductivity only occurs if the interlayer state is occupied.[11] Analysis of pure CaC
6 using a high quality ultraviolet light revealed to conduct angle-resolved photoemission spectroscopy measurements. The opening of a superconducting gap in the π* band revealed a substantial contribution to the total electron–phonon-coupling strength from the π*-interlayer interband interaction.[11]
Reagents in chemical synthesis: KC8
The bronze-colored material KC8 is one of the strongest reducing agents known. It has also been used as a catalyst in polymerizations and as a coupling reagent for aryl halides to biphenyls.[12] In one study, freshly prepared KC8 was treated with 1-iodododecane delivering a modification (micrometre scale carbon platelets with long alkyl chains sticking out providing solubility) that is soluble in chloroform.[12] Another potassium graphite compound, KC24, has been used as a neutron monochromator. A new essential application for potassium graphite was introduced by the invention of the potassium-ion battery. Like the lithium-ion battery, the potassium-ion battery should use a carbon-based anode instead of a metallic anode. In this circumstance, the stable structure of potassium graphite is an important advantage.
See also
- Covalent superconductors
- Magnesium diboride, which uses hexagonal planar boron sheets instead of carbon
- Pyrolytic graphite
References
- 1 2 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0-08-037941-9.
- ↑ H-P Boehm; Setton, R.; Stumpp, E.; et al. (1994). "Nomenclature and terminology of graphite intercalation compounds" (PDF). Pure & Appl. Chem. (PDF). 66 (9): 1893. doi:10.1351/pac199466091893.
- 1 2 Ottmers, D.M.; Rase, H.F. (1966). "Potassium graphites prepared by mixed-reaction technique". Carbon. 4 (1): 125–127. doi:10.1016/0008-6223(66)90017-0. ISSN 0008-6223.
- 1 2 3 4 Catherine E. Housecroft; Alan G. Sharpe (2008). "Chapter 14: The group 14 elements". Inorganic Chemistry, 3rd Edition. Pearson. p. 386. ISBN 978-0-13-175553-6.
- ↑ NIST Ionizing Radiation Division 2001 – Major Technical Highlights. physics.nist.gov
- 1 2 3 Emery, N.; Hérold, Claire; Marêché, Jean-François; Lagrange, Philippe; et al. (2008). "Review: Synthesis and superconducting properties of CaC6". Sci. Technol. Adv. Mater. (PDF). 9 (4): 044102. Bibcode:2008STAdM...9d4102E. doi:10.1088/1468-6996/9/4/044102. PMC 5099629
 .
. - 1 2 Liu, Yuanyue; Merinov, Boris V.; Goddard, William A. (5 April 2016). "Origin of low sodium capacity in graphite and generally weak substrate binding of Na and Mg among alkali and alkaline earth metals". Proceedings of the National Academy of Sciences. 113 (14): 3735–3739. doi:10.1073/pnas.1602473113.
- ↑ Cohn, Adam P (2016). "Ultrafast Solvent-Assisted Sodium Ion Intercalation into Highly Crystalline Few-Layered Graphene". Nano Letters. 16 (1): 543–8. doi:10.1021/acs.nanolett.5b04187. PMID 26618985.
- ↑ Cohn, Adam P (2016). "Durable potassium ion battery electrodes from high-rate cointercalation into graphitic carbons". Journal of Materials Chemistry A. 4 (39): 14954. doi:10.1039/C6TA06797B.
- ↑ Millman, S E; Zimmerman, G O (1983). "Observation of spin glass state in FeCl3: intercalated graphite". Journal of Physics C: Solid State Physics. 16 (4): L89. doi:10.1088/0022-3719/16/4/001.
- 1 2 Csányi; Littlewood, P. B.; Nevidomskyy, Andriy H.; Pickard, Chris J.; Simons, B. D.; et al. (2005). "The role of the interlayer state in the electronic structure of superconducting graphite intercalated compounds". Nature Physics. 1 (1): 42–45. Bibcode:2005NatPh...1...42C. doi:10.1038/nphys119.
- 1 2 Chakraborty, S.; Chattopadhyay, Jayanta; Guo, Wenhua; Billups, W. Edward; et al. (2007). "Functionalization of Potassium Graphite". Angew. Chem. Int. Ed. 46 (24): 4486–8. doi:10.1002/anie.200605175. PMID 17477336.
Further reading
- T. Enoki, M. Suzuki and M. Endo (2003). Graphite intercalation compounds and applications. Oxford University Press. ISBN 0-19-512827-3.
- M.S. Dresselhaus and G. Dresselhaus Review: (1981). "Intercalation compounds of graphite". Advances in Physics. 30 (2): 139. Bibcode:1981AdPhy..30..139D. doi:10.1080/00018738100101367. (187 pages), also reprinted as Dresselhaus, M. S.; Dresselhaus, G. (2002). "Intercalation compounds of graphite". Advances in Physics. 51: 1. Bibcode:2002AdPhy..51....1D. doi:10.1080/00018730110113644.
- D. Savoia; Trombini, C.; Umani-Ronchi, A.; et al. (1985). "Applications of potassium-graphite and metals dispersed on graphite in organic synthesis" (PDF). Pure & Appl. Chem. (PDF). 57 (12): 1887. doi:10.1351/pac198557121887.
- Suzuki, Itsuko S.; Ting-Yu Huang; Masatsugu Suzuki (13 June 2002). "Magnetic phase diagram of the stage-1 CoCl2 graphite intercalation compound: Existence of metamagnetic transition and spin-flop transitions". Phys. Rev. B. 65 (22): 224432. doi:10.1103/PhysRevB.65.224432.
- Rancourt, DG; C Meschi; S Flandrois (1986). "S=1/2 antiferromagnetic finite chains effectively isolated by frustration: CuCl2-intercalated graphite.". Phys Rev B Condens Matter. 33 (1): 347–355. doi:10.1103/PhysRevB.33.347. PMID 9937917.
External links
| Wikimedia Commons has media related to Graphite intercalation compounds. |