Newman projection
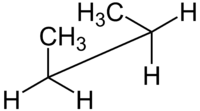
| Molecule of butane in syn-clinal (-sc) conformation | |
 |
 |
| Sawhorse projection |
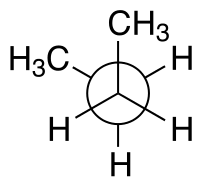
Newman projection |
A Newman projection, useful in alkane stereochemistry, visualizes chemical conformations of a carbon-carbon chemical bond from front to back, with the front carbon represented by a dot and the back carbon as a circle (see below). The front carbon atom is called proximal, while the back atom is called distal. This type of representation is useful for assessing the torsional angle between bonds.
A Sawhorse representation, on the other hand, views a carbon-carbon bond from an oblique angle. This type of representation makes it easy to visualize the molecule as a whole in 3-D.
A Wedge and Dash model views the 3-D shape of a molecule too. It puts the molecule on a page's plane and the bond that is wedged is pointing outwards, in the front of the page, while the dashed bond is pointing backwards, behind the page.
Other methods for depicting molecules are the Fischer projection, the Haworth projection and the Natta projection.
This projection is named after Melvin Spencer Newman, an American chemist of The Ohio State University who introduced it in 1952.
References
- Newman, M. S. Record. Chem. Progr. (Kresge-Hooker Sci. Lib.) 1952, 13, 111.
- Newman, M. S. A notation for the study of certain stereochemical problems. J. Chem. Educ. 1955, 32, 344-347.
- Klein, David R. "Chapter 4 Section 6: Drawing Newman Projections." Organic Chemistry. 2nd ed. N.p.: Wiley, 2012. 158-59. Print.
See also
| Wikimedia Commons has media related to Newman projection. |