Niosome
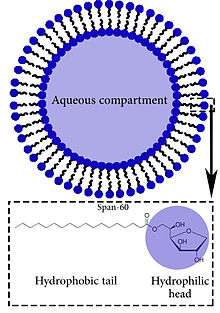
A Niosome is a non-ionic surfactant-based Vesicle (biology and chemistry). Niosomes are formed mostly by non-ionic surfactant and cholesterol incorporation as an excipient.[1] Other excipients can also be used. Niosomes have more penetrating capability than the previous preparations of emulsions.[2] They are structurally similar to liposomes in having a bilayer, however, the materials used to prepare niosomes make them more stable.
Structure
Niosomes are lamellar structures that are microscopic in size. They constitute of non-ionic surfactant of the alkyl or dialkyl polyglycerol ether class and cholesterol with subsequent hydration in aqueous media. The surfactant molecules tend to orient themselves in such a way that the hydrophilic ends of the non-ionic surfactant point outwards, while the hydrophobic ends face each other to form the bilayer. The figure in this article on Niosomes gives a better idea of the lamellar orientation of the surfactant molecules.[3]
Advantages of Niosomes
- Niosomes are osmotically active, chemically stable and have long storage time compared to liposomes
- Their surface formation and modification is very easy because of the functional groups on their hydrophilic heads
- They have high compatibility with biological systems and low toxicity because of their non-ionic nature
- They are biodegradable and non-immunogenic
- They can entrap lipophilic drugs into vesicular bilayer membranes and hydrophilic drugs in aqueous compartments
- They can improve the therapeutic performance of the drug molecules by protecting the drug from biological environment, resulting in better availability and controlled drug delivery by restricting the drug effects to target cells in targeted carriers and delaying clearance from the circulation in sustained drug delivery
- Access to raw materials is convenient [1]
Methods of preparation
Niosomes can be prepared by various methods, including:[1]
- Ether injection method (EIM)
- Hand shaking method (HSM)
- Reverse phase evaporation method (REV)
- trans membrane pH gradient
- the "Bubble" method
- Microfluidization method
- formation of niosomes from proniosomes (Proniosome technology (PT))[4]
- Thin-film hydration method (TFH)
- Heating method (HM)
- Freeze and thaw method (FAT)
- Dehydration rehydration method (DRM)
Methods of preparation in detail: Based on the vesicle size, niosomes can be divided into three groups. These are small unilamellar vesicles (SUV, size=0.025-0.05 μm), multilamellar vesicles (MLV, size=>0.05 μm), and large unilamellar vesicles (LUV, size=>0.10 μm).
Methods of Preparation: Niosomes are prepared by different methods based on the sizes of the vesicles and their distribution, number of double layers, entrapment efficiency of the aqueous phase and permeability of vesicle membrane.
Preparation of small unilamellar vesicles Sonication The aqueous phase containing drug is added to the mixture of surfactant and cholesterol in a scintillation vial.[11] The mixture is homogenised using a sonic probe at 60°C for 3 minutes. The vesicles are small and uniform in size.
Micro fluidisation Two fluidised streams move forward through precisely defined micro channel and interact at ultra-high velocities within the interaction chamber.[21] Here, a common gateway is arranged such that the energy supplied to the system remains within the area of niosomes formation. The result is a greater uniformity, smaller size and better reproducibility.
Preparation of multilamellar vesicles Hand shaking method (Thin film hydration technique) In the hand shaking method, surfactant and cholesterol are dissolved in a volatile organic solvent such as diethyl ether, chloroform or methanol in a rotary evaporator, leaving a thin layer of solid mixture deposited on the wall of the flask.[11] The dried layer is hydrated with aqueous phase containing drug at normal temperature with gentle agitation.
Trans-membrane pH gradient (inside acidic) drug uptake process (remote Loading)'''' Surfactant and cholesterol are dissolved in chloroform.[22] The solvent is then evaporated under reduced pressure to obtain a thin film on the wall of the round-bottom flask. The film is hydrated with 300 mM citric acid (pH 4.0) by vortex mixing. The multilamellar vesicles are frozen and thawed three times and later sonicated. To this niosomal suspension, aqueous solution containing 10 mg/ml of drug is added and vortexed. The pH of the sample is then raised to 7.0-7.2 with 1M disodium phosphate. This mixture is later heated at 60°C for 10 minutes to produce the desired multilamellar vesicles.
Preparation of large unilamellar vesicles Reverse phase evaporation technique (REV) In this method, cholesterol and surfactant are dissolved in a mixture of ether and chloroform.[23] An aqueous phase containing drug is added to this and the resulting two phases are sonicated at 4-5°C. The clear gel formed is further sonicated after the addition of a small amount of phosphate buffered saline. The organic phase is removed at 40°C under low pressure. The resulting viscous niosome suspension is diluted with phosphate-buffered saline and heated in a water bath at 60°C for 10 min to yield niosomes.
Ether injection method The ether injection method is essentially based on slow injection of niosomal ingredients in ether through a 14-gauge needle at the rate of approximately 0.25 ml/min into a preheated aqueous phase maintained at 60°C.[11,24] The probable reason behind the formation of larger unilamellar vesicles is that the slow vapourisation of solvent results in an ether gradient extending towards the interface of aqueous-nonaqueous interface. The former may be responsible for the formation of the bilayer structure. The disadvantages of this method are that a small amount of ether is frequently present in the vesicles suspension and is difficult to remove.
Miscellaneous Multiple membrane extrusion method A mixture of surfactant, cholesterol, and diacetyl phosphate in chloroform is made into thin film by evaporation.[20] The film is hydrated with aqueous drug solution and the resultant suspension extruded through polycarbonate membranes, which are placed in a series for up to eight passages. This is a good method for controlling niosome size.
Niosome preparation using polyoxyethylene alkyl ether The size and number of bilayer of vesicles consisting of polyoxyethylene alkyl ether and cholesterol can be changed using an alternative method.[25] Temperature rise above 60°C transforms small unilamellar vesicles to large multilamellar vesicles (>1 μm), while vigorous shaking at room temperature shows the opposite effect, ie, transformation of multilamellar vesicles into unilamellar ones. The transformation from unilamellar to multilamellar vesicles at higher temperature might be the characteristic for polyoxyethylene alkyl ether (ester) surfactant, since it is known that polyethylene glycol (PEG) and water remix at higher temperature due to breakdown of hydrogen bonds between water and PEG moieties. Generally, free drug is removed from the encapsulated drug by gel permeation chromatography dialysis method or centrifugation method. Often, density differences between niosomes and the external phase are smaller than that of liposomes, which make separation by centrifugation very difficult. Addition of protamine to the vesicle suspension facilitates separation during centrifugation.
Emulsion method The oil in water (o/w) emulsion is prepared from an organic solution of surfactant, cholesterol, and an aqueous solution of the drug.[26,27] The organic solvent is then evaporated, leaving niosomes dispersed in the aqueous phase.
Lipid injection method This method does not require expensive organic phase. Here, the mixture of lipids and surfactant is first melted and then injected into a highly agitated heated aqueous phase containing dissolved drug. Here, the drug can be dissolved in molten lipid and the mixture will be injected into agitated, heated aqueous phase containing surfactant.
Niosome preparation using Micelle Niosomes may also be formed from a mixed micellar solution by the use of enzymes. A mixed micellar solution of C16 G2, dicalcium hydrogen phosphate, polyoxyethylene cholesteryl sebacetate diester (PCSD) converts to a niosome dispersion when incubated with esterases. PCSD is cleaved by the esterases to yield polyoxyethylene, sebacic acid and cholesterol. Cholesterol in combination with C16 G2 and DCP then yields C16 G2 niosomes.
Applications
Niosomes are a novel drug delivery system that are finding application in:
- gene delivery [5]
- drug targeting
- antineoplastic treatment
- leishmaniasis treatment
- delivery of peptide drugs
- studying immune response
- carriers for haemoglobin
- transdermal drug delivery systems[6]
- cosmetics[7]
References
- 1 2 3 4 "Nano-niosomes as nanoscale drug delivery systems: An illustrated review". Journal of Controlled Release. 185: 22–36. doi:10.1016/j.jconrel.2014.04.015.
- ↑ Paracha, Usman Zafar (14 May 2008). "Your Source of Information: Niosomes".
- ↑ PharmaXChange.info - Niosomes Article on niosomes covering topics such as structure, methods of preparation, advantages and applications
- ↑ "PharmaXChange.info - Articles - Niosomes".
- ↑ "A novel cationic niosome formulation for gene delivery to the retina". Journal of Controlled Release. 174: 27–36. doi:10.1016/j.jconrel.2013.11.004.
- ↑ "Niosomes".
- ↑ "Cosmetic and pharmaceutical compositions containing niosomes and a water-soluble polyamide, and a process for preparing these compositions".