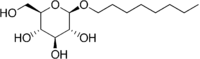
Octyl glucoside
 | |
| Names | |
|---|---|
| IUPAC name
(2R,3S,4S,5R,6R)-2-(hydroxymethyl)-6-octoxyoxane-3,4,5-triol | |
| Other names
n-octyl-β-D-glucoside | |
| Identifiers | |
| 29836-26-8 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 56585 |
| ECHA InfoCard | 100.045.337 |
| EC Number | 249-887-8 |
| MeSH | C018619 |
| PubChem | 62852 |
| |
| |
| Properties | |
| C14H28O6 | |
| Molar mass | 292.37 g/mol |
| Surface tension: | |
| 0.025 M[1] | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Octyl glucoside (n-octyl-β-D-glucoside) is a nonionic surfactant frequently used to solubilise integral membrane proteins for studies in biochemistry. Structurally, it is a glycoside derived from glucose and octanol. Like Genapol X-100 and Triton X-100, it is a nonphysiological amphiphile that makes lipid bilayers less "stiff".[2]
Applications
Octyl glucoside has become one of the most important detergents for purification of membrane proteins because it generally does not denature the protein and can readily be removed from final protein extracts.[3] Above its critical micelle concentration of 0.025 M[1] (~0.7% w/v), it was noted as the best detergent for improving selectivity of immunoprecipitation of phosphotyrosine modified proteins.[4] This detergent has also been shown to rapidly inactivate infective HIV at concentrations above its CMC.[5]
The compound gained popularity with researchers following the publication of an improved synthesis in 1978.[6][7] However, in 1990 the cost remained prohibitive for large-scale protein isolation.[8]
Octyl glucoside has been proposed as a conditioning agent to prevent microbial colonization of contact lenses, due to its ability to lower the hydrophobicity of contact lenses and prevent adhesion of Staphylococcus epidermidis and Pseudomonas aeruginosa.[9]
See also
External links
References
- 1 2 Shinoda, Kozo; Yamaguchi, Tokio; Hori, Ryohei (1961). "The Surface Tension and the Critical Micelle Concentration in Aqueous Solution of β-D-Alkyl Glucosides and their Mixtures". Bulletin of the Chemical Society of Japan. 34 (2): 237–241. doi:10.1246/bcsj.34.237.
- ↑ Lundbaek, Ja; Birn, P; Hansen, Aj; Søgaard, R; Nielsen, C; Girshman, J; Bruno, Mj; Tape, Se; Egebjerg, J; Greathouse, Dv; Mattice, Gl; Koeppe, Re, 2nd; Andersen, Os (May 2004). "Regulation of Sodium Channel Function by Bilayer Elasticity: The Importance of Hydrophobic Coupling. Effects of Micelle-forming Amphiphiles and Cholesterol" (Free full text). The Journal of General Physiology. 123 (5): 599–621. doi:10.1085/jgp.200308996. ISSN 0022-1295. PMC 2234500
 . PMID 15111647.
. PMID 15111647. - ↑ Morandat, S; El, Kirat, K (Apr 2007). "Solubilization of supported lipid membranes by octyl glucoside observed by time-lapse atomic force microscopy". Colloids and surfaces. B, Biointerfaces. 55 (2): 179–84. doi:10.1016/j.colsurfb.2006.11.039. ISSN 0927-7765. PMID 17207975.
- ↑ Zhang, G; Neubert, Ta (Jan 2006). "Use of detergents to increase selectivity of immunoprecipitation of tyrosine phosphorylated peptides prior to identification by MALDI quadrupole-TOF MS". Proteomics. 6 (2): 571–8. doi:10.1002/pmic.200500267. ISSN 1615-9853. PMID 16342243.
- ↑ Bosley A, Marshall HN, Badralmaa Y, Natarajan V (Jun 2008). "A method of HIV-1 inactivation compatible with antibody-based depletion of abundant proteins from plasma.". PROTEOMICS - Clinical Applications. 2 (6): 904–7. doi:10.1002/prca.200780086. PMID 21136887.
- ↑ See PubMed search for "octyl[Title] AND glucoside[Title]" for a timeline of publications.
- ↑ Keana, Jf; Roman, Rb (1978). "Improved synthesis of n-octyl-beta-D-glucoside: a nonionic detergent of considerable potential in membrane biochemistry". Membrane biochemistry. 1 (3–4): 323–7. ISSN 0149-046X. PMID 756493.
- ↑ Kobs, Sf (Nov 1990). "Recovery of octyl beta-glucoside from detergent/protein mixtures". Analytical Biochemistry. 191 (1): 47–9. doi:10.1016/0003-2697(90)90385-M. ISSN 0003-2697. PMID 2077942.
- ↑ Santos, L; Rodrigues, D; Lira, M; Oliveira, R; Real, Oliveira, Me; Vilar, Ey; Azeredo, J (May 2007). "The effect of octylglucoside and sodium cholate in Staphylococcus epidermidis and Pseudomonas aeruginosa adhesion to soft contact lenses". Optometry and vision science : official publication of the American Academy of Optometry. 84 (5): 429–34. doi:10.1097/OPX.0b013e318058a0cc. ISSN 1040-5488. PMID 17502827.