Perbromate
4
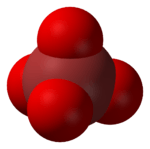
In chemistry, the perbromate ion is the anion having the chemical formula BrO−
4. It is an oxyanion of bromine, the conjugate base of perbromic acid, in which bromine has the oxidation state +7.[1] Unlike its chlorine and iodine analogs (perchlorate and periodate), it is difficult to synthesize.[2] It has tetrahedral molecular geometry.[3]
The term perbromate also refers to a compound that contains the BrO−
4 anion or the –OBrO
3 functional group.
The perbromate ion is a strong oxidizing agent.[2] The reduction potential for the BrO−
4/Br− couple is +0.68 V at pH 14. This is comparable to selenite's reduction potential.
Synthesis
Attempted syntheses of perbromates were unsuccessful until 1968, when it was finally obtained by the beta decay of selenium-83 in a selenate salt:[4][5]
- 83
SeO2−
4 → 83
BrO−
4 + β−
Subsequently, it was successfully synthesized again by the electrolysis of LiBrO
3, although only in low yield.[5][6] Later, it was obtained by the oxidation of bromate with xenon difluoride.[3][7] Once perbromates are obtained, perbromic acid can be produced by protonating BrO−
4.[2]
One effective method of producing perbromate is by the oxidation of bromate with fluorine under alkaline conditions:[2][8]
- BrO−
3 + F
2 + 2 OH−
→ BrO−
4 + 2 F−
+ H
2O
This synthesis is much easier to perform on a large scale than the electrolysis route or oxidation by xenon difluoride.[8]
In 2011 a new, more effective synthesis was discovered: perbromate ions were formed through the reaction of hypobromite and bromate ions in an alkaline sodium hypobromite solution.[9]
Diperiodatonickelate anions in alkaline solution can oxidise bromate to perbromate. This is a relatively lower cost and fluorine free synthesis.[10]
References
- ↑ Egon Wiberg; Nils Wiberg; Arnold Frederick Holleman (2001). Inorganic chemistry. Academic Press. p. 439. ISBN 0-12-352651-5.
- 1 2 3 4 W. Henderson (2000). Main group chemistry (Volume 3 of Tutorial chemistry texts). Royal Society of Chemistry. pp. 136–137. ISBN 0-85404-617-8.
- 1 2 Kurt H. Stern (2001). High temperature properties and thermal decomposition of inorganic salts with oxyanions. CRC Press. p. 224. ISBN 0-8493-0256-0.
- ↑ Appelman, E. H. (1973). "Nonexistent compounds. Two case histories". Accounts of Chemical Research. 6 (4): 113–117. doi:10.1021/ar50064a001.
- 1 2 Appelman, E. H. (1968). "Synthesis of perbromates". Journal of the American Chemical Society. 90 (7): 1900–1901. doi:10.1021/ja01009a040.
- ↑ Kenneth Malcolm Mackay; W. Henderson (2002). Rosemary Ann Mackay, ed. Introduction to modern inorganic chemistry (6th ed.). CRC Press. p. 488. ISBN 0-7487-6420-8.
- ↑ Egon Wiberg; Nils Wiberg; Arnold Frederick Holleman (2001). Inorganic chemistry. Academic Press. p. 395. ISBN 0-12-352651-5.
- 1 2 Appelman, E. H. (1969). "Perbromic acid and perbromates: synthesis and some properties". Inorg. Chem. 8 (2): 223–227. doi:10.1021/ic50072a008.
- ↑ Pisarenko, Aleksey N.; Young, Robert; Quiñones, Oscar; J. Vanderford, Brett; B. Mawhinney, Douglas (2011). "Two New Methods of Synthesis for the Perbromate Ion: Chemistry and Determination by LC-MS/MS". Inorg. Chem. 50 (18): 8691–8693. doi:10.1021/ic201329q.
- ↑ Bilehal, Dinesh C.; Kulkarni, Raviraj M.; Nandibewoor, Sharanappa T. (January 2002). "Kinetics and Mechanism of Oxidation of Bromate by Diperiodatonickelate(IV) in Aqueous Alkaline Medium--A Simple Method for Formation of Perbromate". Inorganic Reaction Mechanisms. 4 (1-2): 103–109. doi:10.1080/1028662021000020244.