Peroxynitric acid
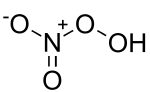 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Hydroxy nitrate | |||
| Systematic IUPAC name | |||
| Identifiers | |||
| 125239-87-4[3] | |||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider | 58833 | ||
| PubChem | 65357 | ||
| |||
| |||
| Properties | |||
| HNO4 | |||
| Molar mass | 79.01224 g/mol | ||
| Related compounds | |||
| Related compounds |
|||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Peroxynitric acid is the chemical compound with the formula HNO
4. It is an oxoacid of nitrogen, after peroxynitrous acid.
Preparation
Peroxynitrate, the conjugate base of peroxynitric acid, is formed rapidly during decomposition of peroxynitrite in neutral conditions.[4]
References
- ↑ "Peroxynitric Acid - Compound Summary".
- ↑ "peroxynitric acid". PubChem. Retrieved 9 December 2012.
- 1 2 "125239-87-4". ChemIndex. Retrieved 9 December 2012.
- ↑ "Direct evidence of singlet molecular oxygen generation from peroxynitrate, a decomposition product of peroxynitrite.". PubMed. Retrieved 9 December 2012.
This article is issued from Wikipedia - version of the 11/24/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.