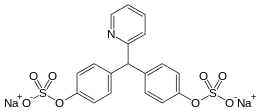
Sodium picosulfate
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code |
A06AB08 (WHO) A06AB58 (WHO) (combinations) |
| Identifiers | |
| |
| CAS Number |
10040-45-6 |
| PubChem (CID) | 68654 |
| ChemSpider |
61910 |
| UNII |
LR57574HN8 |
| ECHA InfoCard | 100.030.097 |
| Chemical and physical data | |
| Formula | C18H13NNa2O8S2 |
| Molar mass | 481.409 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Sodium picosulfate (INN, also known as sodium picosulphate) is a contact stimulant laxative used as a treatment for constipation or to prepare the large bowel before colonoscopy or surgery. It is sold under the trade names Sodipic Picofast, Laxoberal, Laxoberon,[1] Purg-Odan, Picolax, Guttalax, Namilax, Pico-Salax,[2] PicoPrep,[3] and Prepopik,[4] among others.
Effects
Orally administered sodium picosulfate is generally used for thorough evacuation of the bowel, usually for patients who are preparing to undergo a colonoscopy. It takes 12–24 hours to work, since it works in the colon.[4]
The most common side effects of picosulfate are abdominal cramps and diarrhea.
The use of sodium picosulfate has also been associated with certain electrolyte disturbances, such as hyponatremia and hypokalemia.[5] Patients are often required to drink large amounts of clear fluids as well as rehydrate to reestablish the electrolyte balance.
Mechanism of action
Sodium picosulfate is a prodrug.[6] It has no significant direct physiological effect on the intestine, however it is metabolised by gut bacteria into the active compound 4,4'-dihydroxydiphenyl-(2-pyridiyl)methane (DPM, BPHM).[6][7] This compound is a stimulant laxative and increases peristalsis in the gut.[6][8]
Sodium picosulfate is typically prescribed in a combined formulation with magnesium citrate, an osmotic laxative. This combination is a highly effective laxative, often prescribed to patients for bowel cleansing prior to colonoscopies.[6][9]
References
- ↑ Website of Merck Pakistan
- ↑ PICO SALAX Product Information
- ↑ Tjandra, Joe J.; Chan, Miranda; Tagkalidis, Peter P. (2006). "Oral sodium phosphate (Fleet(R)) is a superior colonoscopy preparation to Picoprep(R) (sodium picosulfate-based preparation)". Diseases of the Colon & Rectum. 49 (5): 616–620. doi:10.1007/s10350-005-0323-2. PMID 16525746.
- 1 2 "FDA News Release – FDA approves new colon-cleansing drug for colonoscopy prep". Food and Drug Administration. July 17, 2012. Retrieved December 1, 2016.
- ↑ ADRAC (February 2002). "Electrolyte disturbances with sodium picosulfate bowel cleansing products". Aust Adv Drug React Bull. 21 (1). Free full text from the Australian Therapeutic Goods Administration
- 1 2 3 4 Adamcewicz, Margaret; Bearelly, Dilip; Porat, Gail; Friedenberg, Frank K. (2011-01-01). "Mechanism of action and toxicities of purgatives used for colonoscopy preparation". Expert Opinion on Drug Metabolism & Toxicology. 7 (1): 89–101. doi:10.1517/17425255.2011.542411. ISSN 1742-5255. PMC 3030244
 . PMID 21162694.
. PMID 21162694. - ↑ Forth, W.; Nell, G.; Rummel, W.; Andres, H. (1972-03-01). "The hydragogue and laxative effect of the sulfuric acid ester and the free diphenol of 4,4′-dihydroxydiphenyl-(pyridyl-2)-methane". Naunyn-Schmiedeberg's Archives of Pharmacology. 274 (1): 46–53. doi:10.1007/BF00501005. ISSN 0028-1298.
- ↑ Jauch, R; Hankwitz, R; Beschke, K; Pelzer, H (November 1975). "Bis-(p-hydroxyphenyl)-pyridyl-2-methane: The common laxative principle of Bisacodyl and sodium picosulfate.". Arzneimittel-Forschung. 25 (11): 1796–1800. PMID 1243088.
- ↑ Regev, Arie; Fraser, Gerald; Delpre, George; Leiser, Alfredo; Neeman, Ami; Maoz, Eran; Anikin, Victor; Niv, Yaron. "Comparison of two bowel preparations for colonoscopy: sodium picosulphate with magnesium citrate versus sulphate-free polyethylene glycol lavage solution". The American Journal of Gastroenterology. 93 (9): 1478–1482. doi:10.1111/j.1572-0241.1998.00467.x.