Primary sclerosing cholangitis
| Primary sclerosing cholangitis | |
|---|---|
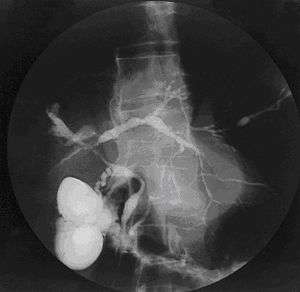 | |
| Cholangiogram of primary sclerosing cholangitis. | |
| Classification and external resources | |
| Specialty | Gastroenterology |
| ICD-10 | K83.0 |
| ICD-9-CM | 576.1 |
| OMIM | 613806 |
| DiseasesDB | 10643 |
| MedlinePlus | 000285 |
| eMedicine | med/3556 |
| Patient UK | Primary sclerosing cholangitis |
| MeSH | D015209 |
Primary sclerosing cholangitis (PSC) is a disease of the bile ducts that causes inflammation and obliterative fibrosis of bile ducts inside and/or outside of the liver. This pathological process impedes the flow of bile to the intestines and can lead to cirrhosis of the liver, liver failure, and other complications, including bile duct and liver cancer. The underlying cause of the inflammation remains unknown, but elements of autoimmunity and microbial dysbiosis have been described[1] and are suggested by the observation that approximately 75% of individuals with PSC also have inflammatory bowel disease (IBD), most often ulcerative colitis.[2] The most definitive treatment for PSC is liver transplantation, though only a fraction of individuals with PSC will ultimately require it.
Signs and symptoms

Many patients with PSC are asymptomatic, but a substantial proportion will have debilitating signs and symptoms of the disease.[3] These may include:
- Pruritus (i.e., itching), which may be severe.
- Severe fatigue (a non-specific symptom often present in liver disease)
- Jaundice and scleral icterus (i.e., yellowing of the white of the eyes)
- Episodes of acute cholangitis (infection within the bile ducts), which can be life-threatening[4]
- Dark urine due to excess conjugated bilirubin, which is water-soluble and excreted by the kidneys (i.e. choluria)
- Malabsorption, especially of fat, and steatorrhea (fatty stool), due to an inadequate amount of bile reaching the small intestine, leading to decreased levels of the fat-soluble vitamins, A, D, E and K.
- Hepatomegaly (i.e., enlarged liver), due to portal hypertension caused by compression of portal veins by the proximate sclerosed intrahepatic bile ducts, and right upper quadrant abdominal pain
- Portal hypertension, a complication of cirrhosis, which can manifest with esophageal and parastomal varices[5] as well as hepatic encephalopathy (mental status alteration/disturbance caused by liver dysfunction and shunting of blood away from the scarred liver; such that ammonia detoxification is reduced with concomitant encephalopathy)
Cause
Primary sclerosing cholangitis is idiopathic (i.e., having, as at present, no known cause). While thought to be an autoimmune disease, it does not demonstrate a clear response to immunosuppressants. Thus, many experts believe it to be a complex, multifactorial (including immune-mediated) disorder and perhaps one that encompasses several different hepatobiliary diseases.[6][7]
Data have provided novel insights suggesting:
- an important association between the intestinal microbiota and PSC[8][9][10][11] and
- a process referred to as cellular senescence and the senescence-associated secretory phenotype (SASP) in the pathogenesis of PSC.[12][13]
In addition, there are longstanding, well-recognized associations between PSC and human leukocyte antigen (HLA) alleles (e.g., A1, B8, and DR3).[1]
Pathophysiology
PSC is characterized by inflammation of the bile ducts (cholangitis) with consequent stricturing (i.e., narrowing) and hardening (sclerosis) of these ducts due to scar formation, be it inside and/or outside of the liver.[14] The resulting scarring of the bile ducts obstructs the flow of bile, which further perpetuates bile duct and liver injury. Chronic impairment of bile flow due to blockage and dysfunctional bile transport (cholestasis) causes progressive biliary fibrosis and ultimately biliary cirrhosis and liver failure.[15]
The primary physiological function of bile is to assist in the breakdown and absorption of fat in the intestinal tract; a relative deficiency of bile can lead to fat malabsorption and deficiencies of fat-soluble vitamins (A, D, E, K).
Diagnosis
PSC is generally diagnosed on the basis of having at least two of three clinical:
- serum alkaline phosphatase (ALP) > 1.5x the upper limit of normal;
- cholangiography demonstrating biliary strictures or irregularity consistent with PSC; and,
- liver histology (if available).
Historically, a cholangiogram would be obtained via endoscopic retrograde cholangiopancreatography (ERCP), which typically reveals "beading" (alternating strictures and dilation) of the bile ducts inside and/or outside the liver. Currently, the preferred option for diagnostic cholangiography, given its non-invasive yet highly accurate nature, is magnetic resonance cholangiopancreatography (MRCP), a magnetic resonance imaging technique. MRCP has unique strengths, including high spatial resolution, and can even be used to visualize the biliary tract of small animal models of PSC.[16]
Most people with PSC have evidence of autoantibodies and abnormal immunoglobulin levels.[17] For example, approximately 80% of people with PSC have perinuclear anti-neutrophil cytoplasmic antibodies; however, this and other immunoglobulin findings are not specific to those with PSC and are of unclear clinical significance/consequence. Antinuclear antibodies and anti-smooth muscle antibody are found in 20%-50% of PSC patients and, likewise, are not specific for the disease but may identify a subgroup of PSC patients who also have autoimmune hepatitis (i.e. PSC-AIH overlap syndrome).[1]
Other markers which may be measured and monitored are a complete blood count, serum liver enzymes, bilirubin levels (usually grossly elevated), kidney function, and electrolytes. Fecal fat measurement is occasionally ordered when symptoms of malabsorption (e.g., gross steatorrhea) are prominent.
The differential diagnosis can include primary biliary cholangitis (formerly referred to as primary biliary cirrhosis), drug induced cholestasis, cholangiocarcinoma, IgG4-related disease, post-liver transplantation non-anastomotic biliary strictures,[18] and HIV-associated cholangiopathy.[19] Primary sclerosing cholangitis and primary biliary cholangitis are distinct entities and exhibit important differences, including the site of tissue damage within the liver, associations with inflammatory bowel disease (IBD), which includes ulcerative colitis and Crohn's disease response to treatment, and risks of disease progression.[20]
Management
No pharmacologic treatment has been approved by the U.S. Food and Drug Administration for PSC. Some experts recommend a trial of ursodeoxycholic acid (UDCA), a bile acid occurring naturally in small quantities in humans, as it has been shown to lower elevated liver enzyme numbers in patients with PSC and has proven effective in other cholestatic liver diseases. However, UDCA has yet to be shown to clearly lead to improved liver histology and adverse event-free survival.[3][21]
Treatment for symptoms of PSC include therapies to relieve itching (antipruritics) (e.g. the bile acid sequestrant (cholestyramine)), antibiotics to treat episodes of acute cholangitis, and vitamin supplements, as people with PSC are often deficient in fat-soluble vitamins (vitamin A, vitamin D, vitamin E, and vitamin K).[22]
In some cases, ERCP with balloon dilation, with or without stenting, may be necessary in order to open major blockages (dominant strictures) in the biliary tree. ERCP and specialized techniques may also be needed to help distinguish between a benign PSC stricture and a bile duct cancer (cholangiocarcinoma)[23]
Liver transplantation is the only proven long-term treatment of PSC, although only a fraction of individuals with PSC will need it. Indications for transplantation include recurrent bacterial cholangitis, decompensated cirrhosis, hepatocellular carcinoma, hilar cholangiocarcinoma, and complications of portal hypertension. Not all patients are candidates for liver transplantation, and some will experience disease recurrence afterward.[6]
Although there is no curative treatment, several clinical trials are underway that aim to slow progression of this liver disease.[24]
Prognosis
Estimated median survival from diagnosis until liver transplant or PSC-related death is 21.3 years.[25] Various models have been developed to help predict survival, but their use is generally best suited for research and not clinical purposes. Recently, normalization of serum alkaline phosphatase has been shown to be an accurate and non-invasive predictor of favorable long-term outcomes.[26]
Related diseases
Primary sclerosing cholangitis is one of the major known risk factors for cholangiocarcinoma,[27] a cancer of the biliary tree, for which the lifetime risk among patients with PSC is 10-15%.[28] This represents a 160-fold greater risk of developing cholangiocarcinoma compared to the general population.[28] Surveillance for cholangiocarcinoma in patients with PSC is encouraged, with some experts recommending annual surveillance with a specialized imaging study and serum markers,[29] although consensus regarding the modality and interval has yet to be established.
PSC is strongly associated with inflammatory bowel disease (IBD), in particular ulcerative colitis (UC) and to a lesser extent Crohn's disease. As many as 5% of patients with IBD are co-diagnosed with PSC[30] and approximately 70% of people with PSC have IBD.[15] Those with both PSC and IBD are at approximately 30-fold increased risk of developing colon cancer; therefore, regular surveillance with high-resolution colonoscopy is recommended.[31] Of note, the presence of colitis appears to be associated with a greater risk of liver disease progression and bile duct cancer (cholangiocarcinoma) development, although this relationship remains poorly understood.[32] Close monitoring of PSC patients is vital.
Other diseases with which PSC is associated include osteoporosis (hepatic osteodystrophy) and hypothyroidism.
Epidemiology
There is a 2-3:1 male-to-female predilection in primary sclerosing cholangitis.[15] PSC can affect men and women at any age, although it is commonly diagnosed in the fourth decade of life, most often in the presence of inflammatory bowel disease (IBD).[14] PSC progresses slowly and is often asymptomatic, so it can be present for years before it is diagnosed and before it causes clinically significant consequences. There is relatively little data on the prevalence and incidence of primary sclerosing cholangitis, with studies in different countries showing annual incidence of 0.068–1.3 per 100,000 people and prevalence 0.22–8.5 per 100,000; given that PSC is closely linked with ulcerative colitis, it is likely that the risk is higher in populations where UC is more common.[33] In the United States, an estimated 25,000 individuals have PSC.
See also
- Chris Klug – professional snowboarder with PSC who had liver transplant
- Chris LeDoux – professional rodeo rider and country musician with PSC who died of cholangiocarcinoma
- Elena Baltacha – British professional tennis player, diagnosed with PSC at age 19 and died five months after being diagnosed with PSC-associated liver cancer (specifically cholangiocarcinoma) at the age of 30.
- Walter Payton – died of complications of PSC.
References
- 1 2 3 Charatcharoenwitthaya P, Lindor KD (Feb 2006). "Primary sclerosing cholangitis: diagnosis and management". Current Gastroenterology Reports. 8 (1): 75–82. doi:10.1007/s11894-006-0067-8. PMID 16510038.
- ↑ Sleisenger, MH (2006). Sleisenger and Fordtran's gastrointestinal and liver disease: pathophysiology, diagnosis, management (8th ed.). Philadelphia: Saunders.
- 1 2 Tabibian JH, Lindor KD (Sep 2014). "Ursodeoxycholic acid in primary sclerosing cholangitis: If withdrawal is bad, then administration is good (right?)". Hepatology. 60 (3): 785–8. doi:10.1002/hep.27180.
- ↑ Tabibian JH, Yang JD, Baron TH, Kane SV, Enders FB, Gostout CJ (2016). "Weekend Admission for Acute Cholangitis Does Not Adversely Impact Clinical or Endoscopic Outcomes". Dig Dis Sci. 61 (1): 53–61. doi:10.1007/s10620-015-3853-z. PMID 26391268. Epub 2015 Sep 21.
- ↑ Tabibian JH, Abu Dayyeh BK, Gores GJ, Levy MJ (2015). "A novel, minimally-invasive technique for management of peristomal varices". Hepatology. doi:10.1002/hep.27925.
- 1 2 Tabiban JH, Lindor KD (Feb 2013). "Primary sclerosing cholangitis: a review and update on therapeutic developments". Expert Rev. Gastroenterol Hepatol. 7 (2): 103–14.
- ↑ O'Hara SP, Tabibian JH, Splinter PL, LaRusso NF (Mar 2013). "The dynamic biliary epithelia: Molecules, pathways, and disease". J Hepatol. 58 (3): 575–82. doi:10.1016/j.jhep.2012.10.011.
- ↑ Tabibian JH, O'Hara SP, Trussoni CE, Tietz PS, Splinter PL, Mounajjed T, Hagey LR, Larusso NF (2015). "Absence of the intestinal microbiota exacerbates hepatobiliary disease in a murine model of primary sclerosing cholangitis". Hepatology. 63: 185–196. doi:10.1002/hep.27927.
- ↑ Tabibian JH, O'Hara SP, Lindor KD (2014). "Primary sclerosing cholangitis and the microbiota: current knowledge and perspectives on etiopathogenesis and emerging therapies". Scand J Gastroenterol. 49 (8): 901–8. doi:10.3109/00365521.2014.913189. PMC 4210190
 . PMID 24990660.
. PMID 24990660. - ↑ Tabibian JH, Varghese C, O'Hara SP, LaRusso NF (2015). "Microbiome-immune interactions and liver disease". Clin Liver Dis. 5 (4): 83–85. doi:10.1002/cld.453.
- ↑ Tabibian JH, Varghese C, LaRusso NF, O'Hara SP (2015). "The Enteric Microbiome in Hepatobiliary Health and Disease". Liver Int. 36: 480–7. doi:10.1111/liv.13009. PMC 4825184
 . PMID 26561779.
. PMID 26561779. - ↑ Tabibian JH, O'Hara SP, Splinter PL, Trussoni CE, Larusso NF (Jun 2014). "Cholangiocyte senescence via N-Ras activation is a characteristic of primary sclerosing cholangitis". Hepatology. 59 (6): 2263–75. doi:10.1002/hep.26993.
- ↑ Tabibian JH, Trussoni CE, O'Hara SP, Splinter PL, Heimbach JK, LaRusso NF (2014). "Characterization of cultured cholangiocytes isolated from livers of patients with primary sclerosing cholangitis". Lab Invest. 94 (10): 1126–33. doi:10.1038/labinvest.2014.94. PMC 4184949
 . PMID 25046437.
. PMID 25046437. - 1 2 Hirschfield, Gideon M; Karlsen, Tom H; Lindor, Keith D; Adams, David H. "Primary sclerosing cholangitis". The Lancet. 382 (9904): 1587–1599. doi:10.1016/s0140-6736(13)60096-3.
- 1 2 3 Robbins SL, Kumar V, Cotran RS (2003). "Chapter 16". Robbins basic pathology (7th ed.). Philadelphia: Saunders. pp. 620–1. ISBN 0-7216-9274-5.
- ↑ Tabibian JH, Macura SI, O'Hara SP, Fidler JL, Glockner JF, Takahashi N, Lowe VJ, Kemp BJ, Mishra PK, Tietz PS, Splinter PL, Trussoni CE, LaRusso NF (2013). "Micro-computed tomography and nuclear magnetic resonance imaging for noninvasive, live-mouse cholangiography". Lab Invest. 93 (6): 733–43. doi:10.1038/labinvest.2013.52. PMC 3875307
 . PMID 23588707.
. PMID 23588707. - ↑ Tabibian JH, Enders F, Imam MH, Kolar G, Lindor KD, Talwalkar JA (2014). "Association between serum IgE level and adverse clinical endpoints in primary sclerosing cholangitis" (PDF). Ann Hepatol. 13 (3): 384–9. PMC 4215553
 . PMID 24756015.
. PMID 24756015. - ↑ Tabibian JH, Asham EH, Goldstein L, Han S, Saab S, Tong MJ, Busuttil R, Durazo FA (2009). "Endoscopic Treatment with Multiple Stents for Post-Liver Transplantation Nonanastomotic Biliary Strictures". Gastrointest Endosc. 69 (7): 1236–1243. doi:10.1016/j.gie.2008.09.057.
- ↑ Lazaridis KN, LaRusso NF (2015). "The Cholangiopathies". Mayo Clin Proc. 90 (6): 791–800. doi:10.1016/j.mayocp.2015.03.017.
- ↑ Trivedi, Palak J.; Corpechot, Christophe; Pares, Albert; Hirschfield, Gideon M. (2016-02-01). "Risk stratification in autoimmune cholestatic liver diseases: Opportunities for clinicians and trialists". Hepatology. 63 (2): 644–659. doi:10.1002/hep.28128. ISSN 1527-3350.
- ↑ Lindor KD, Kowdley KV, Luketic VA, Harrison ME, McCashland T, Befeler AS, Harnois D, Jorgensen R, Petz J, Keach J, Mooney J, Sargeant C, Braaten J, Bernard T, King D, Miceli E, Schmoll J, Hoskin T, Thapa P, Enders F (Sep 2009). "High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis". Hepatology. 50 (3): 671–3. doi:10.1002/hep.23082. PMC 2758780
 . PMID 19585548.
. PMID 19585548. - ↑ Liver, European Association for the Study of the. "EASL Clinical Practice Guidelines: Management of cholestatic liver diseases". Journal of Hepatology. 51 (2): 237–267. doi:10.1016/j.jhep.2009.04.009.
- ↑ Tabibian JH, Visrodia KH, Levy MJ, Gostout CJ (2015). "Advanced endoscopic imaging of indeterminate biliary strictures". World J Gastrointest Endosc. 7 (18): 1268–78. doi:10.4253/wjge.v7.i18.1268.
- ↑ Trivedi, Palak J.; Hirschfield, Gideon M. (2013-05-01). "Treatment of autoimmune liver disease: current and future therapeutic options". Therapeutic Advances in Chronic Disease. 4 (3): 119–141. doi:10.1177/2040622313478646. ISSN 2040-6223. PMC 3629750
 . PMID 23634279.
. PMID 23634279. - ↑ Boonstra, Kirsten; Weersma, Rinse K.; van Erpecum, Karel J.; Rauws, Erik A.; Spanier, B.W. Marcel; Poen, Alexander C.; van Nieuwkerk, Karin M.; Drenth, Joost P.; Witteman, Ben J. (2013-12-01). "Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis". Hepatology. 58 (6): 2045–2055. doi:10.1002/hep.26565. ISSN 1527-3350.
- ↑ Al Mamari S, Djordjevic J, Halliday JS, Chapman RW (Feb 2013). "Improvement of serum alkaline phosphatase to <1.5 upper limit of normal predicts better outcome and reduced risk of cholangiocarcinoma in primary sclerosing cholangitis". J Hepatol. 58 (2): 329–34. doi:10.1016/j.jhep.2012.10.013.
- ↑ Tsaitas C, Semertzidou A, Sinakos E (April 2014). "Update on inflammatory bowel disease in patients with primary sclerosing cholangitis". World J Hepatol. 6 (4): 178–87. doi:10.4254/wjh.v6.i4.178. PMC 4009473
 . PMID 24799986.
. PMID 24799986. - 1 2 Kummen M, Schrumpf E, Boberg KM (August 2013). "Liver abnormalities in bowel diseases". Best Pract Res Clin Gastroenterol. 27 (4): 531–42. doi:10.1016/j.bpg.2013.06.013. PMID 24090940.
- ↑ Tabibian JH, Lindor KD. Challenges of Cholangiocarcinoma Detection in Patients with Primary Sclerosing Cholangitis. J Analytical Oncology. 2012;1(1):50-55.
- ↑ Olsson R, Danielsson A, Järnerot G, et al. (1991). "Prevalence of primary sclerosing cholangitis in patients with ulcerative colitis". Gastroenterology. 100 (5 Pt 1): 1319–23. PMID 2013375.
- ↑ Tabibian JH, Moradkhani A, Topazian MD. Colorectal cancer surveillance in primary sclerosing cholangitis and inflammatory bowel disease. Ann Hepatol. 2015;14(4):564-566.
- ↑ Boonstra, Kirsten; Weersma, Rinse K.; van Erpecum, Karel J.; Rauws, Erik A.; Spanier, B.W. Marcel; Poen, Alexander C.; van Nieuwkerk, Karin M.; Drenth, Joost P.; Witteman, Ben J. (2013-12-01). "Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis". Hepatology. 58 (6): 2045–2055. doi:10.1002/hep.26565. ISSN 1527-3350.
- ↑ Feld JJ, Heathcote EJ (October 2003). "Epidemiology of autoimmune liver disease". J. Gastroenterol. Hepatol. 18 (10): 1118–28. doi:10.1046/j.1440-1746.2003.03165.x. PMID 12974897.
External links
- PSC Partners Seeking a Cure a not-for profit patient support and research sponsorship organization
- Information from The Morgan Foundation for the Study of PSC
- Patient Information from PSC Support, not-for profit patient organization