Plutonium-241
| Plutonium-241 | |
|---|---|
| General | |
| Name, symbol | Plutonium-241,241Pu |
| Neutrons | 147 |
| Protons | 94 |
| Nuclide data | |
| Natural abundance | 0 (Artificial) |
| Half-life | 14 years |
| Decay products | 241Am |
| Actinides and fission products by half-life | ||||||||
|---|---|---|---|---|---|---|---|---|
| Actinides[1] by decay chain | Half-life range (y) |
Fission products of 235U by yield[2] | ||||||
| 4n | 4n+1 | 4n+2 | 4n+3 | |||||
| 4.5–7% | 0.04–1.25% | <0.001% | ||||||
| 228Ra№ | 4–6 | † | 155Euþ | |||||
| 244Cmƒ | 241Puƒ | 250Cf | 227Ac№ | 10–29 | 90Sr | 85Kr | 113mCdþ | |
| 232Uƒ | 238Puƒ№ | 243Cmƒ | 29–97 | 137Cs | 151Smþ | 121mSn | ||
| 248Bk[3] | 249Cfƒ | 242mAmƒ | 141–351 |
No fission products | ||||
| 241Amƒ | 251Cfƒ[4] | 430–900 | ||||||
| 226Ra№ | 247Bk | 1.3 k – 1.6 k | ||||||
| 240Puƒ№ | 229Th№ | 246Cmƒ | 243Amƒ | 4.7 k – 7.4 k | ||||
| 245Cmƒ | 250Cm | 8.3 k – 8.5 k | ||||||
| 239Puƒ№ | 24.1 k | |||||||
| 230Th№ | 231Pa№ | 32 k – 76 k | ||||||
| 236Npƒ | 233Uƒ№ | 234U№ | 150 k – 250 k | ‡ | 99Tc₡ | 126Sn | ||
| 248Cm | 242Puƒ | 327 k – 375 k | 79Se₡ | |||||
| 1.53 M | 93Zr | |||||||
| 237Npƒ№ | 2.1 M – 6.5 M | 135Cs₡ | 107Pd | |||||
| 236U№ | 247Cmƒ | 15 M – 24 M | 129I₡ | |||||
| 244Pu№ | 80 M |
... nor beyond 15.7 M years[5] | ||||||
| 232Th№ | 238U№ | 235Uƒ№ | 0.7 G – 14.1 G | |||||
|
Legend for superscript symbols | ||||||||
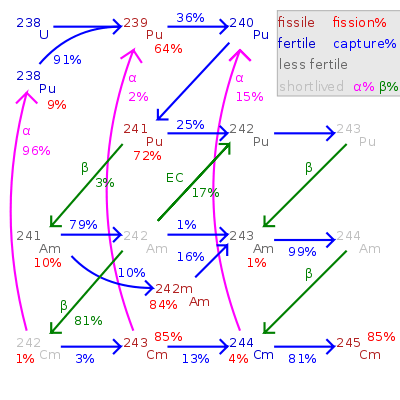
Plutonium-241 (Pu-241) is an isotope of plutonium formed when plutonium-240 captures a neutron. Like Pu-239 but unlike 240Pu, 241Pu is fissile, with a neutron absorption cross section about 1/3 greater than 239Pu, and a similar probability of fissioning on neutron absorption, around 73%. In the non-fission case, neutron capture produces plutonium-242. In general, isotopes with an odd number of neutrons are both more likely to absorb a neutron, and more likely to undergo fission on neutron absorption, than isotopes with an even number of neutrons.
Decay to americium
241Pu has a half-life of 14 years, corresponding to a decay of about 5% of Pu-241 nuclei over a one-year period. The longer spent nuclear fuel waits before reprocessing, the more 241Pu decays to americium-241, which is nonfissile (although fissionable by fast neutrons) and an alpha emitter with a halflife of 432 years which is a major contributor to the radioactivity of nuclear waste on a scale of hundreds or thousands of years.
Americium has lower valence and lower electronegativity than plutonium, neptunium or uranium, so in most nuclear reprocessing, Am tends to fractionate not with U, Np, Pu but with the alkaline fission products: lanthanides, strontium, caesium, barium, yttrium, and is therefore not recycled into nuclear fuel unless special efforts are made.
In a thermal reactor, 241Am captures a neutron to become americium-242, which quickly becomes curium-242 (or, 17.3% of the time, 242Pu) via beta decay. Both Cm-242 and Pu-242 are much less likely to absorb a neutron, and even less likely to fission; however, 242Cm is short-lived (halflife 160 days) and almost always undergoes alpha decay to Pu-238 rather than capturing another neutron. In short, Am-241 needs to absorb two neutrons before again becoming a fissile isotope.
References
- ↑ Plus radium (element 88). While actually a sub-actinide, it immediately precedes actinium (89) and follows a three-element gap of instability after polonium (84) where no isotopes have half-lives of at least four years (the longest-lived isotope in the gap is radon-222 with a half life of less than four days). Radium's longest lived isotope, at 1,600 years, thus merits the element's inclusion here.
- ↑ Specifically from thermal neutron fission of U-235, e.g. in a typical nuclear reactor.
- ↑ Milsted, J.; Friedman, A. M.; Stevens, C. M. (1965). "The alpha half-life of berkelium-247; a new long-lived isomer of berkelium-248". Nuclear Physics. 71 (2): 299. doi:10.1016/0029-5582(65)90719-4.
"The isotopic analyses disclosed a species of mass 248 in constant abundance in three samples analysed over a period of about 10 months. This was ascribed to an isomer of Bk248 with a half-life greater than 9 y. No growth of Cf248 was detected, and a lower limit for the β− half-life can be set at about 104 y. No alpha activity attributable to the new isomer has been detected; the alpha half-life is probably greater than 300 y." - ↑ This is the heaviest isotope with a half-life of at least four years before the "Sea of Instability".
- ↑ Excluding those "classically stable" isotopes with half-lives significantly in excess of 232Th; e.g., while 113mCd has a half-life of only fourteen years, that of 113Cd is nearly eight quadrillion years.