Quebrachitol
 | |
| Names | |
|---|---|
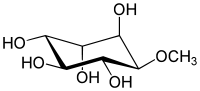
| IUPAC name
(1R,2S,4S,5R)-6-methoxycyclohexane-1,2,3,4,5-pentol | |
| Other names
Quebrachitol L-Quebrachitol (-)-Quebrachitol 2-O-methyl-l-inositol 2-0-methyl-chiro-inositol | |
| Identifiers | |
| 642-38-6 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL501109 |
| ChemSpider | 10254652 |
| PubChem | 151108 |
| |
| |
| Properties | |
| C7H14O6 | |
| Molar mass | 194.18 g/mol |
| Appearance | White to off-white powder |
| Melting point | 190 to 198 °C (374 to 388 °F; 463 to 471 K) |
| Soluble in DMSO, dimethyl formamide, or water | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Quebrachitol is a naturally occurring optically active cyclitol, a cyclic polyol. It can be found in Allophylus edulis[1] and in the serum left after the coagulation of the Hevea brasiliensis latex in the operation of rubber tapping.[2] It is also found in Cannabis sativa,[3] in Paullinia pinnata and in seabuckthorn.[4]
It was first isolated by Tanret in 1887 from the bark of Aspidosperma quebracho. The substance was tested as a sweetening agent for diabetics in 1933. It shows a sweetening property half of that of sucrose but induces colic or diarrhoea at concentration used to render the food palatable.[5]
Quebrachitol is a versatile building block in the construction of naturally occurring bioactive materials.[6] For example, its conversion into antifungal (E)-β-methoxyacrylate, oudemansin X has been made.[7]
References
- ↑ First record of l-quebrachitol in Allophylus edulis (Sapindaceae). Martina Díaz, Andrés González, Ian Castro-Gamboa, David Gonzalez and Carmen Rossini, Carbohydrate Research, Volume 343, Issue 15, 13 October 2008, Pages 2699-2700, doi:10.1016/j.carres.2008.07.014
- ↑ Quebrachitol. Jan van Alphen, Ind. Eng. Chem., 1951, 43 (1), pp 141–145, doi:10.1021/ie50493a041
- ↑ 1955 - ACTA UNIVERSITATIS PALACKIANAE OLOMUCENSIS - TOM. VI. - HEMP AS A MEDICAMENT, Properties of isolated substances. Prof. Jan Kabelik, A brief survey of the methods of isolation and the physical and chemical properties and structures of the isolated antibacterial substances. F. Santavy & Z. Krejci
- ↑ Some new data about antiviral and related activities of seabuckthorn principals and the prospects of their use. Shipulina L.D., All-Russian Research Institute of Medicinal and Aromatic Plants, Moscow, Russia
- ↑ An investigation of quebrachitol as a sweetening agent for diabetics. Robert Alexander McCance and Robert Daniel Lawrence, Biochem J. 1933; 27(4): 986–989
- ↑ Quebrachitol: A Versatile Building Block in the Construction of Naturally Occurring Bioactive Materials. James J. Kiddle, Chem. Rev., 1995, 95 (6), pp 2189–2202, doi:10.1021/cr00038a016
- ↑ Total synthesis of antibiotic (−)-oudemansin X utilizing L-quebrachitol as a chiral pool. Chida N., Yamada K. and Ogawa S., Chemistry Letters, 1992, no4, pp. 687-690
External links
| Look up quebrachitol in Wiktionary, the free dictionary. |
- Quebrachitol on the Sigma-Aldrich website at the Wayback Machine (archived October 3, 2012)