Alpha 1-antichymotrypsin
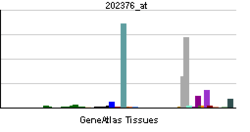
| View/Edit Human | View/Edit Mouse |
Alpha 1-antichymotrypsin (α1AC)[3] is an alpha globulin glycoprotein that is a member of the serpin superfamily. In humans, it is encoded by the SERPINA3 gene.
Function
Alpha 1-antichymotrypsin inhibits the activity of certain enzymes called proteases, such as cathepsin G that is found in neutrophils, and chymases found in mast cells, by cleaving them into a different shape or conformation. This activity protects some tissues, such as the lower respiratory tract, from damage caused by proteolytic enzymes.[4]
This protein is produced in the liver, and is an acute phase protein that is induced during inflammation.
Clinical significance
Deficiency of this protein has been associated with liver disease. Mutations have been identified in patients with Parkinson disease and chronic obstructive pulmonary disease.[5]
Alpha 1-antichymotrypsin is also associated with the pathogenesis of Alzheimer's disease as it enhances the formation of amyloid-fibrils in this disease.[4]
Interactions
Alpha 1-antichymotrypsin has been shown to interact with DNAJC1.[6]
References
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Logan, Carolynn M.; Rice, M. Katherine (1987). Logan's Medical and Scientific Abbreviations. Philadelphia: J. B. Lippincott Company. p. 3. ISBN 0-397-54589-4.
- 1 2 Kalsheker N (1996). "Alpha 1-antichymotrypsin". Int. J. Biochem. Cell Biol. 28 (9): 961–4. doi:10.1016/1357-2725(96)00032-5. PMID 8930118.
- ↑ "Entrez Gene: SERPINA3 serpin peptidase inhibitor, clade A (alpha-1 antiproteinase, antitrypsin), member 3".
- ↑ Kroczynska B, Evangelista CM, Samant SS, Elguindi EC, Blond SY (March 2004). "The SANT2 domain of the murine tumor cell DnaJ-like protein 1 human homologue interacts with alpha1-antichymotrypsin and kinetically interferes with its serpin inhibitory activity". J. Biol. Chem. 279 (12): 11432–43. doi:10.1074/jbc.M310903200. PMC 1553221
 . PMID 14668352.
. PMID 14668352.
Further reading
- Janciauskiene S, Wright HT (1999). "Inflammation, antichymotrypsin, and lipid metabolism: autogenic etiology of Alzheimer's disease.". BioEssays. 20 (12): 1039–46. doi:10.1002/(SICI)1521-1878(199812)20:12<1039::AID-BIES10>3.0.CO;2-Z. PMID 10048303.
- Kalsheker N, Morley S, Morgan K (2002). "Gene regulation of the serine proteinase inhibitors alpha1-antitrypsin and alpha1-antichymotrypsin.". Biochem. Soc. Trans. 30 (2): 93–8. doi:10.1042/BST0300093. PMID 12023832.
External links
- The MEROPS online database for peptidases and their inhibitors: I04.002
- Alpha 1-antichymotrypsin at the US National Library of Medicine Medical Subject Headings (MeSH)