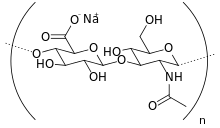
Sodium hyaluronate
 | |
| Clinical data | |
|---|---|
| Trade names | Healon, Provisc, Viscoat, Hyalgan, Euflexxa, Supartz, Gel-One |
| AHFS/Drugs.com | Multum Consumer Information |
| Identifiers | |
| |
| ECHA InfoCard | 100.130.288 |
| | |
Sodium hyaluronate is the sodium salt of hyaluronic acid, a glycosaminoglycan found in various connective, epithelial, and neural tissues. Sodium hyaluronate, a long-chain polymer containing repeating disaccharide units of Na-glucuronate-N-acetylglucosamine, occurs naturally on the corneal endothelium, bound to specific receptors for which it has a high affinity. The polyanionic form, commonly referred to as hyaluronan, is a visco-elastic polymer normally found in the aqueous and vitreous humour.
Medical uses
Sodium hyaluronate for intra-articular injection is used to treat knee pain in patients with osteoarthritis who have not received relief from other treatments. It is similar to the lubricating fluid that occurs naturally in the articular capsule of the knee joint. Once injected into the joint capsule, it acts as both a shock absorber and a lubricant for the joint.[1][2][3] Thus sodium hyaluronate is used as a viscosupplement, administered through a series of injections into the knee, increasing the viscosity of the synovial fluid, which helps lubricate, cushion and reduce pain in the joint.[4] It is generally used as a last resort before surgery[5] and provides symptomatic relief, by recovering the viscoelasticity of the articular fluid, and by stimulating new production from synovial fluid.[6] Use of sodium hyaluronate may reduce the need for joint replacement.[7] Injections appear to increase in effectiveness over the course of four weeks, reaching a peak at eight weeks and retaining some effectiveness at six months, with greater benefit for osteoarthritis than oral analgesics.[8] It may also be effective when used with other joints.[9]
Sodium hyaluronate for intraocular viscoelastic injection is used as a surgical aid in variety of surgical procedures performed on the eyeball including cataract extraction (intra- and extracapsular), intraocular lens implantation, corneal transplant, glaucoma filtration, and retina attachment surgery. It may be used in ophthalmology to assist in the extraction of cataracts, the implantation of intraocular lenses, corneal transplants, glaucoma filtration, retinal attachment and in the treatment of dry eyes.[10] In surgical procedures in the anterior segment of eyeball, instillation of sodium hyaluronate serves to maintain a deep anterior chamber during surgery, allowing for efficient manipulation with less trauma to the corneal endothelium and other surrounding tissues. Its viscoelasticity also helps to push back the vitreous face and prevent formation of a postoperative flat chamber. In posterior segment surgery, sodium hyaluronate serves as a surgical aid to gently separate, maneuver, and hold tissues. It creates a clear field of vision, facilitating intra-operative and post-operative inspection of the retina and photocoagulation.[11]
Sodium hyaluronate injections for plastic surgery are to reduce wrinkles on the face or as a filler in other parts of the body.[12]
Sodium hyaluronate is also used to coat the bladder lining in treating interstitial cystitis.
Contraindications
Sodium hyaluronate is contraindicated for people who are sensitive to hyaluronate preparations, or when there are infections or skin disease at the injection site.
Adverse effects
Adverse effects are relatively rare when used to treat the joints.[6]
When used in ophthalmological procedures, sodium hyaluronate may cause postoperative inflammation, corneal edema or decompensation, and short-term increases in intraocular pressure.
Mechanism of action
Sodium hyaluronate functions as a tissue lubricant and is thought to play an important role in modulating the interactions between adjacent tissues. Sodium hyaluronate is a polysaccharide which is distributed widely in the extracellular matrix of connective tissue in man. It forms a viscoelastic solution in water which makes it suitable for aqueous and vitreous humor in ophthalmic surgery. Mechanical protection for tissues (iris, retina) and cell layers (corneal, endothelium, and epithelium) are provided by the high viscosity of the solution. Elasticity of the solution assists in absorbing mechanical stress and providing a protective buffer for tissues. This viscoelasticity enables maintenance of a deep chamber during surgical manipulation since the solution does not flow out of the open anterior chamber. In facilitating wound healing, it is thought that it acts as a protective transport vehicle, taking peptide growth factors and other structural proteins to a site of action. It is then enzymatically degraded and active proteins are released to promote tissue repair.[13] Sodium hyaluronate is being used intra-articularly to treat osteoarthritis.
Chemistry
Sodium hyaluronate is an ophthalmic agent with viscoelastic properties that is used in joints to supplement synovial fluid.
Pharmacokinetics
Sodium hyaluronate is cleared within hours of injection but appears to have residual effects on contacted cells. It is eliminated via the canal of Schlemm.
History
The first commercially sold sodium hyaluronate was developed by Endre Alexander Balazs under the brand name of Healon, manufactured by Pharmacia AB in Sweden in 1980. Prior to that date this material was used in clinical trials for both human and veterinary (race horses) to treat osteoarthritis in the late 1970s and early 1980s under the brand names of Hylartin and Hylartin Vet.[14] Sodium hyaluronate was subsequently also used to treat osteoarthritis of the knee in year 1986 with the product Hyalart/Hyalgan by Fidia of Italy, in intra-articular injections.[15]
Brand names
Brand names of sodium hyaluronate on the market include (alphabetically):
- Amo Vitrax (ocular)
- Amvisic Plus (ocular)
- Clinitas 0.4% Altacor (eye drop)
- Cystistar, Healon (ocular)
- Euflexxa, Bio Technology General (Israel)-Meditrina SA (Rx articular), Molecular weight: 2,400,000-3,600,000 Daltons
- Eyefill (ocular)
- Gonilert/Verisfield (UK) (Rx/articular). Molecular weight:1,800,000-2,000,000 Daltons
- Hyabak (eye drop)
- Hyalgan/Hyalart- Fidia (Italy)(Medical Device/Rx articular)
- Hylashield (eye drop) Biomatrix, Inc. and I-MED Pharma Inc.
- Hylo-Comod (Eye Drop)
- Hylo Gel (Brazil) (Eye Drop)
- I-Visc (Ocular) I-MED Pharma Inc.
- I-Drop, I-MED Pharma Inc. (eye drop)
- Polyfresh (Eye drop) Orchidia Pharmaceutical Ind
- Monovisc- Anika (USA)(MedicalDevice/articular)
- Oasis Tears (USA) (Eye Drop)
- Olixia Pure (Eye Drop)
- Ostenil- TRB Chemedica (Switzerland)(articular injection)
- Recosyn- Merckle Recordati (Germany)
- Synocrom- Croma Pharma (Austria) (articular injection) . Molecular weight:1,600,000 Daltons
- Viscure- Cube (UK)(Rx/articular), Molecular weight:1,800,000-2,000,000 Daltons
- Viscoseal- TRB Chemedica (Switzerland)(post-arthroscopy synovial fluid replacement)
- Vismed- TRB Chemedica (Switzerland)(eye drop)
- Yardel - Libytec (Impfstoffwerk Dessau-Tornau/Germany,(Rx/articular), Molecular weight:1,800,000-2,000,000 Daltons
References
- ↑ "Hyaluronate sodium: Indications, Side Effects, Warnings" (Web). Drugs.com. Drugs.com. 5 February 2014. Retrieved 25 February 2014.
- ↑ Bannuru RR, Schmid CH, Kent DM, et al. (6 January 2015). "Reviews: Comparative Effectiveness of Pharmacologic Interventions for Knee Osteoarthritis: A Systematic Review and Network Meta-analysis". Annals of Internal Medicine. 162 (1): 46–54. doi:10.7326/M14-1231.
- ↑ Mandl LA, and Losina E (6 January 2015). "Editorials: Relative Efficacy of Knee Osteoarthritis Treatments: Are All Placebos Created Equal?". Annals of Internal Medicine. 162 (1): 71–72. doi:10.7326/M14-2636.
- ↑ Puhl, W.; Scharf, P. (1997). "Intra-articular hyaluronan treatment for osteoarthritis". Annals of the rheumatic diseases. 56 (7): 441. doi:10.1136/ard.56.7.441. PMC 1752402
 . PMID 9486013.
. PMID 9486013. - ↑ Karlsson, J.; Sjögren, L. S.; Lohmander, L. S. (2002). "Comparison of two hyaluronan drugs and placebo in patients with knee osteoarthritis. A controlled, randomized, double-blind, parallel-design multicentre study". Rheumatology (Oxford, England). 41 (11): 1240–1248. doi:10.1093/rheumatology/41.11.1240. PMID 12421996.
- 1 2 Jubb, R. W.; Piva, S.; Beinat, L.; Dacre, J.; Gishen, P. (2003). "A one-year, randomised, placebo (saline) controlled clinical trial of 500-730 kDa sodium hyaluronate (Hyalgan) on the radiological change in osteoarthritis of the knee". International journal of clinical practice. 57 (6): 467–474. PMID 12918884.
- ↑ Kotz, R.; Kolarz, G. (1999). "Intra-articular hyaluronic acid: Duration of effect and results of repeated treatment cycles". American journal of orthopedics (Belle Mead, N.J.). 28 (11 Suppl): 5–7. PMID 10587245.
- ↑ Bannuru, R. R.; Natov, N. S.; Dasi, U. R.; Schmid, C. H.; McAlindon, T. E. (2011). "Therapeutic trajectory following intra-articular hyaluronic acid injection in knee osteoarthritis – meta-analysis". Osteoarthritis and Cartilage. 19 (6): 611–619. doi:10.1016/j.joca.2010.09.014. PMID 21443958.
- ↑ Salk, R. S.; Chang, T. J.; d'Costa, W. F.; Soomekh, D. J.; Grogan, K. A. (2006). "Sodium Hyaluronate in the Treatment of Osteoarthritis of the Ankle: A Controlled, Randomized, Double-Blind Pilot Study". The Journal of Bone and Joint Surgery. 88 (2): 295–302. doi:10.2106/JBJS.E.00193. PMID 16452740.
- ↑ Shimmura, S.; Ono, M.; Shinozaki, K.; Toda, I.; Takamura, E.; Mashima, Y.; Tsubota, K. (1995). "Sodium hyaluronate eyedrops in the treatment of dry eyes". The British journal of ophthalmology. 79 (11): 1007–1011. doi:10.1136/bjo.79.11.1007. PMC 505317
 . PMID 8534643.
. PMID 8534643. - ↑ "Healon (Sodium Hyaluronate)" [package insert]. (2002). Kalamazoo, Michigan: Pharmacia Corporation. (Web). RxList. (Updated 8 December 2004). RxList, Inc. Retrieved 25 February 2014.
- ↑ Beasley, K.; Weiss, M.; Weiss, R. (2009). "Hyaluronic Acid Fillers: A Comprehensive Review". Facial Plastic Surgery. 25 (2): 086–094. doi:10.1055/s-0029-1220647. PMID 19415575.
- ↑ Boucher, W. S.; Letourneau, R.; Huang, M.; Kempuraj, D.; Green, M.; Sant, G. R.; Theoharides, T. C. (2002). "Intravesical sodium hyaluronate inhibits the rat urinary mast cell mediator increase triggered by acute immobilization stress". The Journal of Urology. 167 (1): 380–384. doi:10.1016/S0022-5347(05)65472-9. PMID 11743360.
- ↑ History of Hyaluroan Science, E.A. Balazs, I. Hargittai, M. Hargitti, Hyaluronan, From Basic Science to Clinical Applications Volume 2 Published by Pubmatrix 2011 (ISBN 978-0-9820350-4-7)
- ↑ http://www.fidiapharma.us/en/news-events/2011/fidia-farmaceutici-s-p-a-to-distribute-and-promote-hyalgan-sodium-hyaluronate-for-treatment-of-osteoarthritis,3,13