Sulforaphane
 | |
 | |
 | |
| Names | |
|---|---|
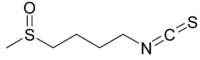
| IUPAC name
1-Isothiocyanato-4-methylsulfinylbutane | |
| Identifiers | |
| 4478-93-7 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:47807 |
| ChEMBL | ChEMBL48802 |
| ChemSpider | 5157 |
| PubChem | 5350 |
| |
| |
| Properties | |
| C6H11NOS2 | |
| Molar mass | 177.29 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Sulforaphane is a compound within the isothiocyanate group of organosulfur compounds. It is obtained from cruciferous vegetables such as broccoli, Brussels sprouts or cabbages. It is produced when the enzyme myrosinase transforms glucoraphanin, a glucosinolate, into sulforaphane upon damage to the plant (such as from chewing), which allows the two compounds to mix and react. Young sprouts of broccoli and cauliflower are particularly rich in glucoraphanin.
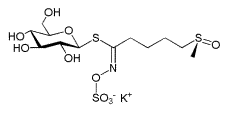 glucoraphanin, glucosinolate precursor to sulforaphane |
Occurrence and isolation
Sulforaphane was identified in broccoli sprouts, which, of the cruciferous vegetables, have the highest concentration of sulforaphane.[1] It is also found in Brussels sprouts, cabbage, cauliflower, bok choy, kale, collards, Chinese broccoli, broccoli raab, kohlrabi, mustard, turnip, radish, arugula, and watercress.
Research
Basic research on sulforaphane includes how it may affect neurodegenerative disorders, cancer, spinal cord injury or gastric diseases,[2][3][4][5] and preliminary human research on autism.[6] As of 2015, clinical trials are being conducted with sulforaphane for autism spectrum disorder.[7]
See also
References
- ↑ Zhang Y, Talalay P, Cho CG, Posner GH (March 1992). "A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure". Proc. Natl. Acad. Sci. U.S.A. 89 (6): 2399–2403. doi:10.1073/pnas.89.6.2399. PMC 48665
 . PMID 1549603.
. PMID 1549603. - ↑ Tarozzi A, Angeloni C, Malaguti M, Morroni F, Hrelia S, Hrelia P (2013). "Sulforaphane as a potential protective phytochemical against neurodegenerative diseases". Oxid Med Cell Longev (Review). 2013: 415078. doi:10.1155/2013/415078. PMC 3745957
 . PMID 23983898.
. PMID 23983898. - ↑ Grabacka MM, Gawin M, Pierzchalska M (2014). "Phytochemical modulators of mitochondria: the search for chemopreventive agents and supportive therapeutics". Pharmaceuticals (Basel) (Review). 7 (9): 913–42. doi:10.3390/ph7090913. PMC 4190497
 . PMID 25192192.
. PMID 25192192. - ↑ Koushki D, Latifi S, Javidan AN, Matin M (June 2014). "Efficacy of some non-conventional herbal medications (sulforaphane, tanshinone IIA, and tetramethylpyrazine) in inducing neuroprotection in comparison with interleukin-10 after spinal cord injury: A meta-analysis". J Spinal Cord Med (Meta-analysis). 38: 13–22. doi:10.1179/2045772314Y.0000000215. PMID 24969510.
- ↑ Moon JK, Kim JR, Ahn YJ, Shibamoto T (2010). "Analysis and anti-Helicobacter activity of sulforaphane and related compounds present in broccoli ( Brassica oleracea L.) sprouts". J. Agric. Food Chem. 58 (11): 6672–7. doi:10.1021/jf1003573. PMID 20459098.
- ↑ Singh, Kanwaljit; Connors, Susan L.; Macklin, Eric A.; Smith, Kirby D.; Fahey, Jed W.; Talalay, Paul; Zimmerman, Andrew W. (28 October 2014). "Sulforaphane treatment of autism spectrum disorder (ASD)". Proceedings of the National Academy of Sciences. 111 (43): 15550–15555. doi:10.1073/pnas.1416940111. ISSN 0027-8424. PMC 4217462
 . PMID 25313065.
. PMID 25313065. - ↑ "Sulforaphane Treatment of Children With Autism Spectrum Disorder (ASD)". clinicaltrials.gov. Retrieved 3 May 2016.