Cetirizine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Zyrtec |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a698026 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | R06AE07 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | well absorbed |
| Protein binding | ~93% |
| Metabolism | Excreted mainly unchanged |
| Biological half-life | 8.3 Hours |
| Excretion | Urine (mainly), hepatic or excrement (Small amounts) |
| Identifiers | |
| |
| Synonyms | Alatrol, Alerid, Alzene, Cetirin, Cetirizin, Cetirizina, Cetzine, Cezin, Histazine, Humex, Letizen, Reactine, Razene, Zetop, Zirtec, Zirtek, Zodac, Zyllergy, Zynor, Zyrlek, Zyrtec |
| CAS Number |
83881-51-0 |
| PubChem (CID) | 2678 |
| IUPHAR/BPS | 1222 |
| DrugBank |
DB00341 |
| ChemSpider |
2577 |
| UNII |
YO7261ME24 |
| KEGG |
D07662 |
| ChEBI |
CHEBI:3561 |
| ChEMBL |
CHEMBL1000 |
| ECHA InfoCard | 100.223.545 |
| Chemical and physical data | |
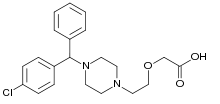
| Formula | C21H25ClN2O3 |
| Molar mass | 388.89 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Cetirizine /sɛˈtɪrᵻziːn/ (trade names Zirtec, Zyrtec, Reactine) is a second-generation antihistamine used in the treatment of hay fever, allergies, angioedema, and urticaria.[1] It is a major metabolite of hydroxyzine, and a racemic selective H1 receptor antagonist.
Second-generation antihistamines like cetirizine are less able to cross the blood-brain barrier and therefore have diminished effects on the central nervous system compared to first-generation drugs: for instance they are less likely to induce drowsiness or to interfere with memory formation.
Medical uses
Allergies
Cetirizine's primary indication is for hay fever and other allergies. Because the symptoms of itching and redness in these conditions are caused by histamine acting on the H1 receptor, blocking those receptors temporarily relieves those symptoms.
Cetirizine is also commonly prescribed to treat acute and (in particular cases) chronic urticaria, more efficiently than any other second-generation antihistamine.
Rhinovirus infection
Interleukin 6 and interleukin 8 have been shown to be elevated in acute respiratory distress syndrome.[2] Cetirizine contains L- and D-stereoisomers. Chemically, levocetirizine is the active L-enantiomer of cetirizine. One recent study of airway epithelial cells showed that levocetirizine may have beneficial effects on the pathophysiologic changes related to human rhinovirus (HRV) infection.[3]
Kimura's disease
Cetirizine is an effective agent in treating the symptoms of Kimura's disease which predominantly affects the lymph nodes and soft tissue of the head and neck in the form of tumor-like lesions. Cetirizine's properties of being effective both in the treatment of pruritus (itching) and as an anti-inflammatory agent make it suitable for the treatment of the pruritus associated with these lesions.[4] In a 2005 study, the American College of Rheumatology conducted treatments initially using prednisone, followed by steroid dosages and azathioprine, omeprazole, and calcium and vitamin D supplements over the course of two years.[4] The skin condition of the patient began to improve and the skin lesions lessened. However, there were symptoms of cushingoid and hirsutism observed before the patient was removed from the courses of steroids and placed on 10 mg/day of cetirizine to prevent skin lesions;[4] an agent suitable for the treatment of pruritus associated with such lesions.[4] Asymptomatically, the patient's skin lesions disappeared after treatment with cetirizine, blood eosinophil counts became normal,[4] corticosteroid effects were resolved,[4] and a remission began within a period of two months.[4] It is also thought that the inhibition of eosinophils may be the key to treatment of Kimura's disease due to the role of eosinophils, rather than other cells with regards to the lesions of the skin.[4]
Availability

Formerly prescription-only in the US and Canada, cetirizine is now available over-the-counter (OTC) in both countries as Zyrtec and Reactine, respectively.[5] Zyrtec was the highest-grossing new non-food product of 2008 in the US, generating sales of $315.9 million.[6] It is also available as a generic drug. In Turkey, Iran, Australia and New Zealand (in the latter of which it is marketed as Razene), Zyrtec is available over-the-counter in pharmacies, and in the UK, Norway and The Netherlands cetirizine can be sold in limited quantities off-the-shelf in any outlet and is often available in supermarkets. In 2009, Germany made many generic drugs containing cetirizine available in pharmacies without prescription.[7] Sweden, Finland, Poland, and Israel also classify cetirizine as an over-the-counter medicine.[8]
In Ireland, cetirizine is known as Zirtek (as well as under generic names), and is available OTC in limited quantities (usually no more than seven tablets per pack).
Like many other antihistamine medications, cetirizine is commonly prescribed in combination with pseudoephedrine hydrochloride, a decongestant. These combinations are marketed using the same brand name as the cetirizine with a "-D" suffix (Zyrtec-D, Virlix-D, etc.)
Adverse effects
Commonly reported side effects of cetirizine include headache (16%), dry mouth (5.7%), and drowsiness (5–20%) or fatigue (5.6%).[9]
Pharmacology
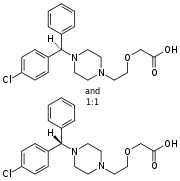
Pharmacodynamics
Cetirizine crosses the blood–brain barrier only slightly, reducing the sedative side-effect common with older antihistamines.[10] It has also been shown to inhibit eosinophil chemotaxis and LTB4 release. At a dosage of 20 mg, Boone et al. found that it inhibited the expression of VCAM-1 in patients with atopic dermatitis.[11] Unlike many other antihistamines, Cetirizine does not exhibit anticholinergic properties.[12]
The levorotary enantiomer of cetirizine, known as levocetirizine, is the more active form.
Pharmacokinetics
Chewable, non-chewable, and syrup forms of cetirizine are similarly absorbed rapidly and effectively, with absorbed food minutely affecting the absorption rate which yields a peak serum level one hour after administration;[13] in a study of healthy volunteers prescribed 10 mg tablets, once daily for 10 days, a mean peak serum level of 311 ng/mL was observed.[14] The metabolic effects of cetirizine are long-acting, remaining in the system for a maximum of 21 hours before being excreted; the average elimination half-life is 8 hours. About 70% of the drug is removed through urination, of which half is observed as unchanged cetirizine compound. Another 10% is excreted.[13][14]
Chemistry
Synthesis
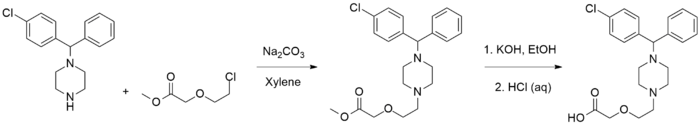 Cetirizine synthesis:[15]
Cetirizine synthesis:[15]
The 1-[(4-chlorophenylmethyl]-piperazine is alkylated with methyl (2-chloroethoxy)-acetate in the presence of sodium carbonate and xylene solvent to produce the Sn2 substitution product in 28% yield. Saponification of the acetate ester is done by refluxing with potash in absolute ethanol to afford a 56% yield of the potassium salt intermediate. This is then hydrolyzed with aqueous HCl and extracted to give a 81% yield of the carboxylic acid product.
See also
References
- ↑ http://www.medscape.com/viewarticle/561316
- ↑ Chollet-Martin, S; Montravers, P; Gibert, C; Elbim, C; Desmonts, JM; Fagon, JY; Gougerot-Pocidalo, MA (1993). "High levels of interleukin-8 in the blood and alveolar spaces of patients with pneumonia and adult respiratory distress syndrome". Infection and immunity. 61 (11): 4553–9. PMC 281204
 . PMID 8406851.
. PMID 8406851. - ↑ Jang YJ, Wang JH, Kim JS, Kwon HJ, Yeo NK, Lee BJ (March 2009). "Levocetirizine inhibits rhinovirus-induced ICAM-1 and cytokine expression and viral replication in airway epithelial cells". Antiviral Res. 81 (3): 226–33. doi:10.1016/j.antiviral.2008.12.001. PMID 19110001.
- 1 2 3 4 5 6 7 8 Ben-Chetrit E, Amir G, Shalit M (February 2005). "Cetirizine: An effective agent in Kimura's disease". Arthritis Rheum. 53 (1): 117–8. doi:10.1002/art.20908. PMID 15696573.
- ↑ Payne, January W (2008-01-09). "Over-the-Counter Zyrtec: A Money-Saver?". U.S. News & World Report.
- ↑ Elliott, Stuart (24 March 2009). "A Strategy When Times Are Tough: 'It's New!'". The New York Times. Retrieved 26 March 2009.
- ↑ de:Cetirizin
- ↑ "Cetirizin". Archived from the original on 2009-03-31.
- ↑ "Zyrtec Side Effects". drugs.com. Drugs.com. Retrieved 21 August 2015.
- ↑ Gupta, A; Chatelain P; Massingham R; Jonsson EN; Hammarlund-Udenaes M (February 2006). "Brain distribution of cetirizine enantiomers: comparison of three different tissue-to-plasma partition coefficients: K(p), K(p,u), and K(p,uu)". Drug Metab. Dispos. 34 (2): 318–23. doi:10.1124/dmd.105.007211. PMID 16303872.
- ↑ Boone M, Lespagnard L, Renard N, Song M, Rihoux JP (July 2000). "Adhesion molecule profiles in atopic dermatitis vs. allergic contact dermatitis: pharmacological modulation by cetirizine". J Eur Acad Dermatol Venereol. 14 (4): 263–6. doi:10.1046/j.1468-3083.2000.00017.x. PMID 11204513. Retrieved 2009-11-19.
- ↑ Orzechowski, RF; DS Currie; CA Valancius (4 January 2005). "Comparative anticholinergic activities of 10 histamine H1 receptor antagonists in two functional models.". European Journal of Pharmacology. 506 (3): 257–264. doi:10.1016/j.ejphar.2004.11.006. PMID 15627436.
- 1 2 Anderson, Philip; Knoben, James E.; Troutman, William G. (2002). Handbook of clinical drug data. New York: McGraw-Hill. p. 807. ISBN 0-07-136362-9.
- 1 2 "Zyrtec prescribing information" (PDF). May 2006. Archived from the original (PDF) on 2010-01-04. Retrieved 2009-11-19.
- ↑ US patent 4525358, Baltes E, De Lannoy J, Rodriguez L, "2-[4-(Diphenylmethyl)-1-piperazinyl]-acetic acids and their amides", issued 1985-06-25, assigned to UCB Pharmaceuticals, Inc.
Books and journals
- Anderson, P. O., Knoben, J. E., et al. (2002) Handbook of clinical drug data 10th ed. McGraw-Hill International
- Pfizer Inc., et al. (2006) ZYRTEC (cetirizine hydrochloride) Tablets, Chewable Tablets and Syrup For Oral Use Pfizer Incorporated publications
- Chetrit, E. B., Amir, G., Shalit, M. (2005). Cetirizine: an effective agent in Kimura's Disease Arthritis & Rheumatism (Arthritis care & research) Vol 53, p117-118
External links
- US FDA approves Zyrtec-D for over the counter sales
- US FDA approves Zyrtec for over the counter sales
- U.S. National Library of Medicine: Drug Information Portal - Cetirizine