Cyanogen fluoride
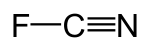 | |
| | |
| Names | |
|---|---|
| IUPAC name
Carbononitridic fluoride[1] | |
| Identifiers | |
| 1495-50-7 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 120749 |
| PubChem | 137036 |
| |
| |
| Properties | |
| CNF | |
| Molar mass | 45.0158 g mol−1 |
| Appearance | Colorless gas |
| Density | 1.026 g mL−1 |
| Boiling point | −46 °C (−51 °F; 227 K) |
| Thermochemistry | |
| Std molar entropy (S |
225.40 J K−1 mol−1 |
| Std enthalpy of formation (ΔfH |
35.98 kJ mol−1 |
| Hazards | |
| EU classification (DSD) |
|
| Related compounds | |
| Related compounds |
|
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cyanogen fluoride is an inorganic compound of carbon, nitrogen, and fluorine. It is a toxic gas at room temperature. It is used in organic synthesis.
This compound may be prepared by pyrolysis of cyanuric fluoride (C3N3F3):[2]
- C3N3F3 → 3 CNF
References
- ↑ "Cyanogen fluoride - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 27 March 2005. Identification. Retrieved 6 June 2012.
- ↑ Fawcett, F. S.; Lipscomb, R. D. (1964). J. Am. Chem. Soc. 86 (13): 2576. doi:10.1021/ja01067a011. Missing or empty
|title=(help)
| Salts and covalent derivatives of the cyanide ion | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCN | He | ||||||||||||||||||
| LiCN | Be(CN)2 | B | C | NH4CN | OCN−, -NCO |
FCN | Ne | ||||||||||||
| NaCN | Mg(CN)2 | Al(CN)3 | SiCN | P(CN)3 | SCN−, -NCS, (SCN)2, S(CN)2 |
ClCN | Ar | ||||||||||||
| KCN | Ca(CN)2 | Sc(CN)3 | Ti(CN)4 | VO(CN)3 | Cr(CN)3 | Mn(CN)2 | Fe(CN)3, Fe(CN)64+, Fe(CN)63+ |
Co(CN)2, Co(CN)3 |
Ni(CN)2 Ni(CN)42− |
CuCN | Zn(CN)2 | Ga(CN)3 | Ge | As(CN)3 | SeCN− (SeCN)2 Se(CN)2 |
BrCN | Kr | ||
| RbCN | Sr(CN)2 | Y(CN)3 | Zr(CN)4 | Nb | Mo | Tc | Ru | Rh | Pd(CN)2 | AgCN | Cd(CN)2 | In(CN)3 | Sn | Sb | Te(CN)2, Te(CN)4 |
ICN | XeCN | ||
| CsCN | Ba(CN)2 | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg2(CN)2, Hg(CN)2 |
TlCN | Pb(CN)2 | Bi(CN)3 | Po | At | Rn | |||
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La | Ce(CN)3, Ce(CN)4 |
Pr | Nd | Pm | Sm | Eu | Gd(CN)3 | Tb | Dy | Ho | Er | Tm | Yb | Lu | |||||
| Ac | Th | Pa | UO2(CN)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||
This article is issued from Wikipedia - version of the 7/4/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.