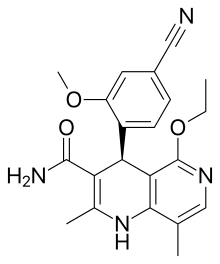
Finerenone
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | none |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| Synonyms | BAY 94-8862 |
| CAS Number | 1050477-31-0 |
| PubChem (CID) | 60150535 |
| ChemSpider | 28669387 |
| UNII | DE2O63YV8R |
| KEGG | D10633 |
| ChEMBL | CHEMBL2181927 |
| Chemical and physical data | |
| Formula | C21H22N4O3 |
| Molar mass | 378.42 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
Finerenone (INN, USAN) (developmental code name BAY-94-8862) is a non-steroidal antimineralocorticoid that is in phase III clinical trials for the treatment of chronic heart failure as of October 2015. It has less relative affinity to other steroid hormone receptors than currently available antimineralocorticoids such as eplerenone and spironolactone, which should result in fewer adverse effects like gynaecomastia, impotence, and low sex drive.[1][2]
Pharmacology
Finerenone blocks mineralocorticoid receptors, which makes it a potassium-sparing diuretic.
This table compares inhibitory (blocking) concentrations (IC50, unit: nM) of three antimineralocorticoids. Mineralocorticoid receptor inhibition is responsible for the desired action of the drugs, whereas inhibition of the other receptors potentially leads to side effects. Lower values mean stronger inhibition.[1]
| Spironolactone | Eplerenone | Finerenone | |
|---|---|---|---|
| Mineralocorticoid receptor | 24 | 990 | 18 |
| Glucocorticoid receptor | 2400 | 22,000 | >10,000 |
| Androgen receptor | 77 | 21,200 | >10,000 |
| Progesterone receptor | 740 | 31,200 | >10,000 |
The above-listed drugs have insignificant affinity for the estrogen receptor.
Chemistry
Unlike currently marketed antimineralocorticoids, finerenone is not a steroid but a dihydropyridine derivative.
Research
The drug is also being investigated in early trials for the treatment of diabetic nephropathy.[3]
See also
References
- 1 2 Schubert-Zsilavecz, M, Wurglics, M, Neue Arzneimittel Herbst 2015 (German)
- ↑ Pitt, B; Anker, S. D.; Böhm, M; Gheorghiade, M; Køber, L; Krum, H; Maggioni, A. P.; Ponikowski, P; Voors, A. A.; Zannad, F; Nowack, C; Kim, S. Y.; Pieper, A; Kimmeskamp-Kirschbaum, N; Filippatos, G (2015). "Rationale and design of MinerAlocorticoid Receptor antagonist Tolerability Study-Heart Failure (ARTS-HF): A randomized study of finerenone vs. Eplerenone in patients who have worsening chronic heart failure with diabetes and/or chronic kidney disease". European Journal of Heart Failure. 17 (2): 224–32. doi:10.1002/ejhf.218. PMID 25678098.
- ↑ Bakris, G. L.; Agarwal, R; Chan, J. C.; Cooper, M. E.; Gansevoort, R. T.; Haller, H; Remuzzi, G; Rossing, P; Schmieder, R. E.; Nowack, C; Kolkhof, P; Joseph, A; Pieper, A; Kimmeskamp-Kirschbaum, N; Ruilope, L. M.; Mineralocorticoid Receptor Antagonist Tolerability Study–Diabetic Nephropathy (ARTS-DN) Study Group (2015). "Effect of Finerenone on Albuminuria in Patients with Diabetic Nephropathy: A Randomized Clinical Trial". JAMA. 314 (9): 884–94. doi:10.1001/jama.2015.10081. PMID 26325557.