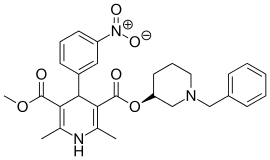
Benidipine
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | C08CA15 (WHO) |
| Identifiers | |
| |
| CAS Number |
105979-17-7 |
| PubChem (CID) | 656668 |
| ChemSpider |
571013 |
| UNII |
4G9T91JS7E |
| ChEMBL |
CHEMBL2218858 |
| Chemical and physical data | |
| Formula | C28H31N3O6 |
| Molar mass | 505.562 |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Benidipine (INN), also known as Benidipinum or benidipine hydrochloride, is a dihydropyridine calcium channel blocker for the treatment of high blood pressure (hypertension). It is a triple L-, T-, and N-type calcium channel blocker. It is reno- and cardioprotective.
Dosing
Benidipine is dosed as 2–8 mg once daily.[1]
Availability
Benidipine is sold as Coniel by Kyowa Hakko Kogyo.
Benidipine is only licensed for use in Japan and selected Southeast Asian countries, where it is sold as 4 mg tablets.
Mechanism
Benidipine is a calcium channel blocker.
Benidipine has additionally been found to act as an antagonist of the mineralocorticoid receptor, or as an antimineralocorticoid.[2]
References
- ↑ Hi-Eisai Pharmaceutical, Inc. "Coniel (benidipine) package insert (Philippines)". MIMS Philippines. CMPMedica. Retrieved 2008-03-31.
- ↑ Luther, James M. (2014). "Is there a new dawn for selective mineralocorticoid receptor antagonism?". Current Opinion in Nephrology and Hypertension. 23 (5): 456–461. doi:10.1097/MNH.0000000000000051. ISSN 1062-4821.
This article is issued from Wikipedia - version of the 9/30/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.