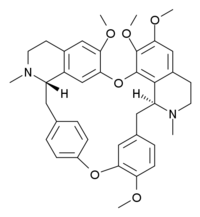
Tetrandrine
 | |
| Names | |
|---|---|
| IUPAC name
9,20,21,25-tetramethoxy-15,30-dimethyl-7,23-dioxa-15,30-diazaheptacyclo[22.6.2.2³,⁶.1⁸,¹².1¹⁴,¹⁸.0²⁷,³¹.0²²,³³]hexatriaconta-3,5,8(34),9,11,18,20,22(33),24(32),25,27(31),35-dodecaene | |
| Identifiers | |
| 518-34-3 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL176045 |
| ECHA InfoCard | 100.208.615 |
| PubChem | 73078 |
| UNII | 29EX23D5AJ |
| |
| Properties | |
| C38H42N2O6 | |
| Molar mass | 622.74988 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Tetrandrine a bis-benzylisoquinoline alkaloid, is a calcium channel blocker. It has anti-inflammatory, immunologic and antiallergenic effects. It inhibits the degranulation of mast cells. It has a "Quinidine like" anti-arrhythmic effect. It has been isolated from Stephania tetrandra S Moore,[1] and other Chinese and Japanese herbs. It has vasodilatory properties and can therefore reduce blood pressure.[2] Tetrandrine may have potential use for the treatment of liver disease[3] and liver cancer.[4][5][6] Tetrandrine has potential therapeutic value to prevent excess scarring/fibrosis in conjunctiva following trabeculectomy or in patients with severe conjunctival inflammation.[7] Tetrandrine has anti-inflammatory and anti-fibrogenic actions, which make tetrandrine and related compounds potentially useful in the treatment of lung silicosis, liver cirrhosis, and rheumatoid arthritis.[8] Tetrandrine has also been shown to inhibit entry of Ebola virus into host cells in vitro and showed therapeutic efficacy against Ebola in preliminary studies on mice.[9]
Biosynthesis
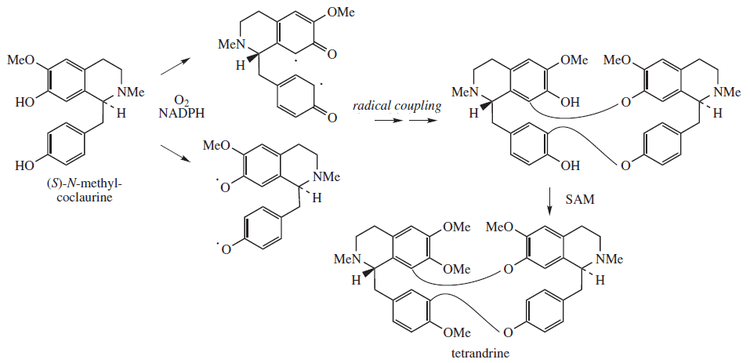
Tetrandrine is biosynthesized from a free radical coupled dimerization of S-N-methylcoclaurine:[10]
Synonyms
Synonyms include fanchinine, fanchinine, hanfangchin A, NSC 77037, (S,S)-(+)-tetrandrine, sinomenine A, TTD, tetrandrin, and d-tetrandrine.[11]
References
- ↑ Zhang L, Geng Y, Duan W, Wang D, Fu M, Wang X.,"Ionic liquid-based ultrasound-assisted extraction of fangchinoline and tetrandrine from Stephaniae tetrandrae. J Sep Sci. 2009 Oct;32(20):3550-4
- ↑ Kwan CY, Achike FI.,"Tetrandrine and related bis-benzylisoquinoline alkaloids from medicinal herbs: cardiovascular effects and mechanisms of action." Acta Pharmacol Sin. 2002 Dec;23(12):1057-68.
- ↑ Feng D, Mei Y, Wang Y, Zhang B, Wang C, Xu L.,"Tetrandrine protects mice from concanavalin A-induced hepatitis through inhibiting NF-kappaB activation." Immunol Lett. 2008 Dec 22;121(2):127-33
- ↑ Liu C, Gong K, Mao X, Li W.,"Tetrandrine induces apoptosis by activating reactive oxygen species and repressing Akt activity in human hepatocellular carcinoma." Int J Cancer. 2011 Sep 15;129(6):1519-31
- ↑ Cheng Z, Wang K, Wei J, Lu X, Liu B"Proteomic analysis of anti-tumor effects by tetrandrine treatment in HepG2 cells." Phytomedicine. 2010 Nov;17(13):1000-5
- ↑ Zhong Yao Cai. .,"[The study of anti-tumor effect of Tetrandrine combined with Nedaplatin on human liver cancer cell line 7402]" 2008 Oct;31(10):1522-5 Authors: Deng WY, Luo SX, Zhou MQ, Li N, Chen XB, Han LL
- ↑ Kitano A, Yamanaka O, Ikeda K, Ishida-Nishikawa I, Okada Y, Shirai K, Saika S"Tetrandrine suppresses activation of human subconjunctival fibroblasts in vitro." Curr Eye Res. 2008 Jul;33(7):559-65
- ↑ Kwan CY, Achike FI.,"Tetrandrine and related bis-benzylisoquinoline alkaloids from medicinal herbs: cardiovascular effects and mechanisms of action." Acta Pharmacol Sin. 2002 Dec;23(12):1057-68.
- ↑ Sakurai Y, et al. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science 2015;347(6225):995-998. DOI 10.1126/science.1258758
- ↑ Dewan S. Bhakuni, Sudha Jain, Awadhesh N. Singh. "Biosynthesis of the bisbenzylisoquinoline alkaloid, tetrandrine" Phytochemistry (1980); 19(11):2347–2350
- ↑ Tetrandrine(Fanchinine) | CAS 518-34-3 | calcium channel Inhibitor | Fanchinine, Hanfangchin A, NSC 77037, S,S-(+)-Tetrandrine, Sinomenine A, TTD, Tetrandrin, d...