Glossary of chemistry terms
 |
| Chemistry |
|---|
This page is a glossary of chemistry terms. Chemistry has an extensive vocabulary and a significant amount of jargon. This is a list of chemical terms, including laboratory tools, glassware, and equipment. Chemistry itself is a physical science concerned with the composition, structure, and properties of matter, as well as the changes it undergoes during chemical reactions.
A
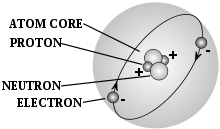
An atom, with protons, neutrons, and electrons labelled.
- absolute zero – a theoretical condition concerning a system at zero Kelvin where a system does not emit or absorb energy (all atoms are at rest)
- absorbance -
- accuracy – how close a value is to the actual or true value; also see precision
- acid – a compound that, when dissolved in water, gives a pH of less than 7.0 or a compound that donates a hydrogen ion
- acid anhydride – a compound with two acyl groups bound to a single oxygen atom
- acid dissociation constant – an equilibrium constant for the dissociation of a weak acid
- actinides – the fifteen chemical elements that are between actinium (89) and lawrencium (103)
- activated complex – a structure that forms because of a collision between molecules while new bonds are formed
- activation energy – the minimum energy that must be input to a chemical system
- activity series
- actual yield
- addition reaction – within organic chemistry, when two or more molecules combine to make a larger one
- adhesion -
- aeration – the mixing of air into a liquid or solid
- alcohol -
- aldehyde -
- alkali metals – the metals of Group 1 on the periodic table
- alkaline earth metals – the metals of Group 2 on the periodic table
- alkane -
- alkene -
- alkyl group -
- alkyne -
- allomer – a substance that has different composition than another, but has the same crystalline structure
- allotropy – elements that can have different structures (and therefore different forms), such as Carbon (diamonds, graphite, and fullerene)
- amplitude – the maximum distance that the particles of the medium carrying the wave move away from their rest position
- anion – negatively charge ions
- anode – the positive side of a dry cell battery or a cell
- aromaticity – chemical property of conjugated rings that results in unusual stability. See also benzene.
- atom – a chemical element in its smallest form, and is made up of neutrons and protons within the nucleus and electrons circling the nucleus
- atomic mass unit
- atomic number – the number representing an element which corresponds with the number of protons within the nucleus
- atomic orbital – the region where the electron of the atom may be found
- atomic radius
- average atomic mass
- Avogadro's law
- Avogadro's number – is the number of particles in a mole of a substance now let's talk of compound
B
- barometer – a device used to measure the pressure in the atmosphere
- base – a substance that accepts a proton and has a high pH; a common example is sodium hydroxide (NaOH)
- base anhydride – oxides of group I and II metal elements
- beat – a slow oscillation in amplitude of a complex wave
- Beer-Lambert law -
- biochemistry – the chemistry of organisms
- Bohr model -
- boiling – the phase transition of liquid vaporizing
- boiling point – the temperature in which the substance starts to boil
- boiling-point elevation – the process where the boiling point is elevated by adding a substance
- bond – the attraction and repulsion between atoms and molecules that is a cornerstone of chemistry
- Boyle's law – for a given mass of gas at constant temperature, the volume varies inversely with the pressure
- Bragg's law -
- Brønsted-Lowrey acid – A chemical species that donates a proton
- Brønsted–Lowry acid–base reaction -
- Brønsted-Lowrey base – A chemical species that accepts a proton
- buckminsterfullerene -
- buffered solution – An aqueous solution consisting of a weak acid and its conjugate base or a weak base and its conjugate acid that resists changes in pH when strong acids or bases are added
- bumping -
- burette (also buret) – glassware used to dispense specific amounts of liquid when precision is necessary (e.g. titration and resource dependent reactions)
C

An example of combustion
- calorimeter -
- catalyst – a chemical compound used to change the rate (either to speed up or slow down) of a reaction, but is regenerated at the end of the reaction.
- cation – positively charged ion
- centrifugation -
- centrifuge – equipment used to separate substances based on density by rotating the tubes around a centred axis
- cell potential – the force in a galvanic cell that pulls electron through reducing agent to oxidizing agent
- chain reaction -
- Charles's law -
- chelation -
- chemical formula -
- chemical law – certain rules that pertain to the laws of nature and chemistry – examples
- chemical reaction – the change of one or more substances into another or multiple substances
- closed system -
- colligative properties -
- colloid – mixture of evenly dispersed substances, such as many milks
- combustion – an exothermic reaction between an oxidant and fuel with heat and often light
- compression – an area in a longitudinal wave where the particles are closer and pushed in
- compound – a substance that is made up of two or more chemically bonded elements
- condensation – the phase change from gas to liquid
- conductor – material that allows electric flow more freely
- conjugate acid -
- conjugated system -
- cooling curve -
- covalent bond – chemical bond that involves sharing electrons
- crest – the highest point in a wave
- crystal – a solid that is packed with ions, molecules or atoms in an orderly fashion
- cuvette – glassware used in spectroscopic experiments. It is usually made of plastic, glass or quartz and should be as clean and clear as possible
D
- Dalton's law of partial pressures -
- deionization – the removal of ions, and in water's case mineral ions such as sodium, iron and calcium
- deliquescence – substances that absorb water from the atmosphere to form liquid solutions
- density – The amount of mass per unit volume. d = m/V
- deposition – settling of particles within a solution or mixture
- diffusion -
- dipolar bond -
- dipole – electric or magnetic separation of charge
- dipole moment – the polarity of a polar covalent bond
- dissolution or solvation – the spread of ions in a monosaccharide
- double bond – sharing of two pairs of electrons (in a covalent bond)
- diatomic-consistent of two atoms
E

Microcentrifuge or Eppendorf tube with Coomassie Blue solution
- earth metal – see alkaline earth metal
- electrolyte – a solution that conducts a certain amount of current and can be split categorically as weak and strong electrolytes
- electrochemical cell – using a chemical reaction's current, electromotive force is made
- electromagnetic radiation – a type of wave that can go through vacuums as well as material and classified as a self-propagating wave
- electromagnetism – fields that have electric charge and electric properties that change the way that particles move and interact
- electromotive force – a device that gains energy as electric charges pass through it
- electron – a subatomic particle with a net charge that is negative
- electron shells – an orbital around the atom's nucleus that has a fixed number electrons (usually two or eight)
- electric charge – a measured property (coulombs) that determine electromagnetic interaction
- element – an atom that is defined by its atomic number
- energy – A system's ability to do work
- enthalpy – measure of the total energy of a thermodynamic system (usually symbolized as H)
- entropy – the amount of energy not available for work in a closed thermodynamic system (usually symbolized as S)
- enzyme – a protein that speeds up (catalyses) a reaction
- Empirical Formula – also called the simplest formula, gives the simplest whole -number ratio of atoms of each element present in a compound.
- eppendorf tube – generalized and trademarked term used for a type of tube; see microcentrifuge
- exothermic process -
- extrinsic property -
F
- freezing – phase transition from liquid to solid
- Faraday constant – a unit of electrical charge widely used in electrochemistry and equal to ~ 96,500 coulombs.
- It represents 1 mol of electrons, or the Avogadro number of electrons: 6.022 × 1023 electrons. F = 96 485.339 9(24) C/mol
- Faraday's law of electrolysis – a two part law that Michael Faraday published about electrolysis
- the mass of a substance altered at an electrode during electrolysis is directly proportional to the quantity of electricity transferred at that electrode
- the mass of an elemental material altered at an electrode is directly proportional to the element's equivalent weight.
- Fick's laws of diffusion -
- frequency – number of cycles per unit of time. Unit: 1 hertz = 1 cycle per 1 second
G
- Galvanic cell – battery made up of electrochemical with two different metals connected by salt bridge
- gas – particles that fill their container though have no definite shape or volume
- Gay-Lussac's Law – The expression Gay-Lussac's law is used for each of the two relationships named after the French chemist Joseph Louis Gay-Lussac and which concern the properties of gases, though it is more usually applied to his law of combining volumes
- geochemistry – the chemistry of and chemical composition of the Earth
- Gibbs energy – value that indicates the spontaneity of a reaction (usually symbolized as G)
- "gram-atom"-one gram atom of an element means collection of 6.023X10^23 atoms
- group – the elements in a column of the periodic table. A family of elements
H
- halogens – Group 17 on the Periodic Table and are all non-metals
- hadron – a subatomic particle of a type including the baryons and mesons that can take part in the strong interaction
- heat – energy transferred from one system to another by thermal interaction
- Henry's law -
- Hess's law -
- Hund's rules -
I
- ideal gas -
- ideal gas law – the volume of a gas is proportional to the amount of gas and its Kelvin temperature and inversely proportional to its pressure
- indicator – a special compound added to solution that changes color depending on the acidity of the solution; different indicators have different colors and effective pH ranges
- induced radioactivity – radioactivity caused by bombarding a stable isotope with elemental particles, forming a radioactive isotope
- inorganic compound – compounds that do not contain carbon, though there are exceptions (see main article)
- inorganic chemistry – a part of chemistry concerned with inorganic compounds
- IUPAC – International Union of Pure and Applied Chemistry
- insulator – material that resists the flow of electric current
- intrinsic property -
- ion – a molecule that has gained or lost one or more electrons
- ionic bond – electrostatic attraction between oppositely charged ions
- ionization -The breaking up of a compound into separate ions.
J
- jodium – Latin name of the halogen element iodine
- Joule – The SI unit of energy, defined as a newton-meter.
K
- kelvin – A unit of measure for temperature based upon an absolute scale.
- ketone – an organic compound with a carbonyl group between two carbon atoms
- Kinetics – A sub-field of chemistry specializing in reaction rates
- Kinetic energy – The energy of an object due to its motion.
L
- lanthanides – Elements 57 through 71
- lattice – Unique arrangement of atoms or molecules in a crystalline liquid or solid.
- Laws of thermodynamics
- Lewis acid -
- Lewis base -
- liquid – A state of matter in which cohesive force is less than or equal to the separating force.
- light – Portion of the electromagnetic spectrum which is visible to the naked eye. Also called "visible light."
- London dispersion forces – A weak intermolecular force
- laws of motion – An object in motion stays in motion; an object at rest stays at rest unless an unbalanced force acts on it.
M

This is a molecule, which is one of the key components within chemistry
- magnetic quantum number -
- manometer - Instrument to measure pressure, invented by Evangelista Torricelli in 1643.
- mass -
- mass concentration -
- mass fraction -
- metal – Chemical element that is a good conductor of both electricity and heat and forms cations and ionic bonds with non-metals.
- melting – The phase change from a solid to a liquid
- metalloid – A substance possessing both the properties of metals and non-metals
- methylene blue – a heterocyclic aromatic chemical compound with the molecular formula C16H18N3SCl
- microcentrifuge – a small plastic container that is used to store small amounts of liquid
- molar attenuation coefficient -
- molar mass -
- mole – abbreviated mol – a measurement of an amount of substance; a single mole contains approximately 6.022×1023 units or entities
- a mole of water contains 6.022×1023 H2O molecules
- molecular formula -
- molecular orbital – region where an electron can be found in a molecule (as opposed to an atom)
- molecular orbital diagram -
- molecule – a chemically bonded number of atoms that are electrically neutral
N
- neat – conditions with a liquid reagent or gas performed with no added solvent or cosolvent
- neutron – a neutral unit or subatomic particle that has no net charge
- neutrino – a particle that can travel at speeds close to the speed of light and are created as a result of radioactive decay
- nucleus – the centre of an atom made up of neutrons and protons, with a net positive charge
- noble gases – group 18 elements, those whose outer electron shell is filled
- non-metal – an element which is not metallic
- nuclear – of or pertaining to the atomic nucleus
- nuclear magnetic resonance spectroscopy – technique that exploits the magnetic properties of certain nuclei, useful for identifying unknown compounds
- number density – a measure of concentration of countable objects (atoms, molecules, etc.) in space; number per volume
O
- orbital – may refer to either an atomic orbital or a molecular orbital
- organic compound – compounds that contain carbon
- organic chemistry – a part of chemistry concerned with organic compounds
- organic redox reaction -
- oxidation -
- oxidation state -
- oxidizing agent -
P
- pH – the measure of acidity (or basicity) of a solution
- plasma – state of matter similar to gas in which a certain portion of the particles are ionized
- other metal – metallic elements in the p-block, characterized by having a combination of relatively low melting points (all less than 950 K) and relatively high electronegativity values (all more than 1.6, revised Pauling)
- potential energy – energy stored in a body or in a system due to its position in a force field or due to its configuration
- precipitate – formation of a solid in a solution or inside another solid during a chemical reaction or by diffusion in a solid
- precision – How close the results of multiple experimental trials are. See also accuracy.
- photon – a carrier of electromagnetic radiation of all wavelength (such as gamma rays and radio waves)
- proton – a positive unit or subatomic particle that has a positive charge
- protonation – the addition of a proton (H+) to an atom, molecule, or ion
Q
- Quantum mechanics – the study of how atoms, molecules, subatomic particles, etc. behave and are structured
- quarks – elementary particle and a fundamental constituent of matter
- quanta- It is the minimum amount of bundle of energy
R
- radiation – energy in the form of waves or subatomic particles when there is a change from high energy to low energy states
- radioactive decay – the process of an unstable atomic nucleus losing energy by emitting radiation
- Raoult's law -
- reactivity series -
- reagent -
- redox -
- reducing agent -
- reduction potential -
S
- s-block elements – Group 1 and 2 elements (alkali and alkaline metals), which includes Hydrogen and Helium
- salts – ionic compounds composed of anions and cations
- salt bridge – devices used to connection reduction with oxidation half-cells in an electrochemical cell
- saline solution – general term for NaCl in water
- Schrödinger equation – quantum state equation which represents the behaviour of an election around an atom
- semiconductor – an electrically conductive solid that is between a conductor and an insulator
- single bond – sharing of one pair of electrons
- sol – a suspension of solid particles in liquid. Artificial examples include sol-gels.
- solid – one of the states of matter, where the molecules are packed close together, there is a resistance of movement/deformation and volume change; see Young's modulus
- solute – the part of the solution that is mixed into the solvent (NaCl in saline water)
- solution – homogeneous mixture made up of multiple substances. It is made up of solutes and solvents.
- solvent – the part of the solution that dissolves the solute (H2O in saline water)
- spectrochemistry -
- spectroscopy – study of radiation and matter, such as X-ray absorption and emission spectroscopy
- speed of light – the speed of anything that has zero rest mass (Energyrest = mc² where m is the mass and c is the speed of light)
- Standard conditions for temperature and pressure or SATP – a standardisation used in order compare experimental results (25 °C and 100.000 kPa)
- state of matter – matter having a homogeneous, macroscopic phase; gas, plasma, liquid, and solid are the most well known (in increasing concentration)
- structural formula -
- sublimation – a phase transition from solid to limewater fuel or gas
- subatomic particles – particles that are smaller than an atom; examples are protons, neutrons and electrons
- substance – material with definite chemical composition
T
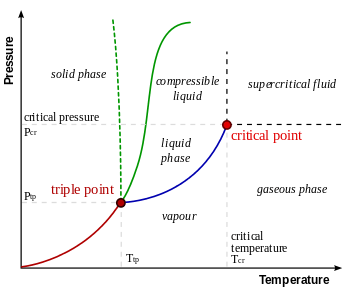
Phase diagram showing the triple and critical points of a substance
- talc – a mineral representing the one on the Mohs Scale and composed of hydrated magnesium silicate with the chemical formula H2Mg3(SiO3)4 or Mg3Si4O10(OH)2
- temperature – the average energy of microscopic motions of particles
- theoretical yield – see yield
- theory – a model describing the nature of a phenomenon
- thermal conductivity – a property of a material to conduct heat (often noted as )
- thermochemistry – the study of absorption/release of heat within a chemical reaction
- thermodynamics – the study of the effects of changing temperature, volume or pressure (or work, heat, and energy) on a macroscopic scale
- thermodynamic stability – when a system is in its lowest energy state with its environment (equilibrium)
- thermometer – device that measures the average energy of a system
- titration – the process of titrating one solution with another, also called volumetric analysis
- torr – a unit to measure pressure (1 Torr is equivalent to 133.322 Pa or 1.3158×10−3 atm)
- transition metal – elements that have incomplete d sub-shells, but also may be referred to as the d-block elements
- transuranic element – element with atomic number greater than 92; none of the transuranic elements are stable
- triple bond – the sharing of three pairs of electrons within a covalent bond (example N2)
- triple point – the place where temperature and pressure of three phases are the same (Water has a special phase diagram)
- Tyndall effect – the effect of light scattering by colloidal (mixture where one substance is dispersed evenly through another) or suspended particles
U
- UN number – a four digit code used to note hazardous and flammable substances
- uncertainty – a characteristic that any measurement that involves estimation of any amount cannot be exactly reproducible
- Uncertainty principle – knowing the location of a particle makes the momentum uncertain, while knowing the momentum of a particle makes the location uncertain
- unit cell – the smallest repeating unit of a lattice
- unit factor – statements used in converting between units
- universal or ideal gas constant – proportionality constant in the ideal gas law (0.08206 L·atm/(K·mol))
V
- valence electron – the outermost electrons of an atom, which are located in electron shells
- Valence bond theory – a theory explaining the chemical bonding within molecules by discussing valencies, the number of chemical bonds formed by an atom
- valency – The combining capacity of an element.
- van der Waals force – one of the forces (attraction/repulsion) between molecules
- van 't Hoff factor – ratio of moles of particles in solution to moles of solute dissolved
- vapor – when a substance is below the critical temperature while in the gas phase
- vapour pressure – pressure of vapour over a liquid at equilibrium
- vaporization – phase change from liquid to gas
- viscosity – the resistance of a liquid to flow (oil)
- volt – one joule of work per coulomb – the unit of electrical potential transferred
- voltmeter – instrument that measures the cell potential
- volumetric analysis – see titration
W
- water – H2O – a chemical substance, a major part of cells and Earth, and covalently bonded
- wave function – a function describing the electron's position in a three-dimensional space
- work – the amount of force over distance and is in terms of joules (energy)
X
- X-ray – form of ionizing, electromagnetic radiation, between gamma and UV rays
- X-ray diffraction – a method for establishing structures of crystalline solids using singe wavelength X-rays and looking at diffraction pattern
- X-ray photoelectron spectroscopy – a spectroscopic technique to measure composition of a material
Y
- yield – the amount of product produced during a chemical reaction
Z
- zone melting – a way to remove impurities from an element by melting it and slowly travel down an ingot (cast)
- Zwitterion – is a chemical compound whose net charge is zero and hence is electrically neutral. But there are some positive and negative charges in it, due to the formal charge, owing to the partial charges of its constituent atoms.
- zinc-an element or a metal with symbol Zn
See also
- Chemistry
- List of chemical elements
- Glossary of areas of mathematics
- Glossary of astronomy
- Glossary of biology
- Glossary of engineering
- Glossary of physics
- Glossary of probability and statistics
External links
| Wikibooks has a book on the topic of: Chemistry |
| Wikiquote has quotations related to: English_chemistry_mnemonics |
This article is issued from Wikipedia - version of the 11/25/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.
