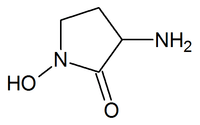
HA-966
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | 1003-51-6 |
| PubChem (CID) | 1232 |
| ChemSpider | 1195 |
| UNII |
F2JLV9220T |
| ECHA InfoCard | 100.162.446 |
| Chemical and physical data | |
| Formula | C4H8N2O2 |
| Molar mass | 116.11972 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
HA-966 or (±) 3-Amino-1-hydroxy-pyrrolidin-2-one is a molecule used in scientific research as a glycine receptor and NMDA receptor antagonist / low efficacy partial agonist. It has neuroprotective and anticonvulsant,[1] anxiolytic,[2] antinociceptive[3] and sedative / hypnotic[4] effects in animal models. Pilot human clinical trials in the early 1960s showed that HA-966 appeared to benefit patients with tremors of extrapyramidal origin.[4]
The two enantiomers of HA-966 have differing pharmacological activity. The glycine/N-methyl-D-aspartate receptor antagonist activity is specific to the R-(+) enantiomer, whereas the sedative and ataxic effects are specific to the S-(-) enantiomer.[5]
R-(+)-HA-966 did not induce drug-appropriate responding in animals trained to discriminate phencyclidine (PCP) from saline, suggesting that the glycine receptor ligand R-(+)-HA-966 has a significantly different behavioral profile than drugs affecting the ion channel of the NMDA receptor complex.[6]
S-(−)-HA-966 has been described as a "γ-hydroxybutyric acid (GHB)-like agent"[7] and a "potent y-butyrolactone-like sedative",[5] but it shows no affinity for the GABAB receptor (GABABR).[7]
See also
References
- ↑ Vartanian MG, Taylor CP (Nov 1991). "Different stereoselectivity of the enantiomers of HA-966 (3-amino-1-hydroxy-2-pyrrolidinone) for neuroprotective and anticonvulsant actions in vivo.". Neuroscience Letters. 133 (1): 109–12. doi:10.1016/0304-3940(91)90069-6. PMID 1838797.
- ↑ Dunn RW, Flanagan DM, Martin LL, Kerman LL, Woods AT, Camacho F, Wilmot CA, Cornfeldt ML, Effland RC, Wood PL, et al. (Apr 1992). "Stereoselective R-(+) enantiomer of HA-966 displays anxiolytic effects in rodents.". European Journal of Pharmacology. 214 (2-3): 207–14. doi:10.1016/0014-2999(92)90120-S. PMID 1355434.
- ↑ Näsström J, Karlsson U, Post C (Feb 1992). "Antinociceptive actions of different classes of excitatory amino acid receptor antagonists in mice.". European Journal of Pharmacology. 212 (1): 21–9. doi:10.1016/0014-2999(92)90067-E. PMID 1313371.
- 1 2 Bonta IL, De Vos CJ, Grijsen H, Hillen FC, Noach EL, Sim AW (Nov 1971). "1-Hydroxy-3-amino-pyrrolidone-2(HA-966): a new GABA-like compound, with potential use in extrapyramidal diseases." (PDF). British Journal of Pharmacology. 43 (3): 514–35. doi:10.1111/j.1476-5381.1971.tb07182.x. PMC 1665789
 . PMID 5157720.
. PMID 5157720. - 1 2 Singh L, Donald AE, Foster AC, Hutson PH, Iversen LL, Iversen SD, Kemp JA, Leeson PD, Marshall GR, Oles RJ, et al. (Jan 1990). "Enantiomers of HA-966 (3-amino-1-hydroxypyrrolid-2-one) exhibit distinct central nervous system effects: (+)-HA-966 is a selective glycine/N-methyl-D-aspartate receptor antagonist, but (−)-HA-966 is a potent gamma-butyrolactone-like sedative." (PDF). Proc Natl Acad Sci USA. 87 (1): 347–51. doi:10.1073/pnas.87.1.347. PMC 53260
 . PMID 2153294.
. PMID 2153294. - ↑ Singh L, Menzies R, Tricklebank MD (Sep 1990). "The discriminative stimulus properties of (+)-HA-966, an antagonist at the glycine/N-methyl-D-aspartate receptor.". European Journal of Pharmacology. 186 (1): 129–32. doi:10.1016/0014-2999(90)94069-A. PMID 2149338.
- 1 2 Morrow BA, Lee EJ, Taylor JR, Elsworth JD, Nye HE, Roth RH (Nov 1997). "(S)-(−)-HA-966, a gamma-hydroxybutyrate-like agent, prevents enhanced mesocorticolimbic dopamine metabolism and behavioral correlates of restraint stress, conditioned fear and cocaine sensitization." (PDF). J Pharmacol Exp Ther. 283 (2): 712–21. PMID 9353390.