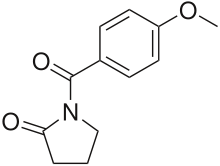
Aniracetam
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Ampamet, Memodrin, Pergamid |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | N06BX11 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Biological half-life | 1-2.5 hours |
| Identifiers | |
| |
| CAS Number |
72432-10-1 |
| PubChem (CID) | 2196 |
| IUPHAR/BPS | 4133 |
| DrugBank |
DB04599 |
| ChemSpider |
2111 |
| UNII |
5L16LKN964 |
| KEGG |
D01883 |
| ChEBI |
CHEBI:47943 |
| ChEMBL |
CHEMBL36994 |
| Chemical and physical data | |
| Formula | C12H13NO3 |
| Molar mass | 219.237 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Aniracetam (Draganon, Sarpul, Ampamet, Memodrin, Referan), also known as N-anisoyl-2-pyrrolidinone, is an ampakine nootropic of the racetam chemical class purported to be considerably more potent than piracetam. It is lipid-soluble and has possible cognition-enhancing effects. It has been tested in animals extensively, Alzheimer's patients, and temporarily impaired healthy subjects. It has shown potential as an anxiolytic in three clinical animal models. It is sold in Europe as a prescription drug,[1] but it is not approved by the Food and Drug Administration for use in the United States.
Pharmacology
Aniracetam has also been shown to positively modulate the AMPA receptor[2] and was used as the parent compound to derive a class of drugs known as the ampakines that are being investigated as nootropics and neuroprotective drugs for the treatment of Alzheimer's disease and other neurodegenerative conditions.[3]
After a confirmed test of the anxiolytic efficacy in a mouse model, haloperidol, mecamylamine, and ketanserin were applied to determine the pathways aniracetam depends on to exert its anti-anxiety effects. Haloperidol completely reversed the anxiolytic effects, and mecamylamine and ketanserin nearly completely reversed the effects. This shows that aniracetam's anxiolytic mechanism is possibly mediated through D2, nACh, or 5-HT2A receptor activity.[4]
The main metabolite of aniracetam (70-80%), N-anisoyl-GABA, reproduces many of the effects of aniracetam.[5][6] 2-Pyrrolidinone and p-anisic acid are additional metabolites of the drug (20-30%), both of which are also active.[6]
Synthesis
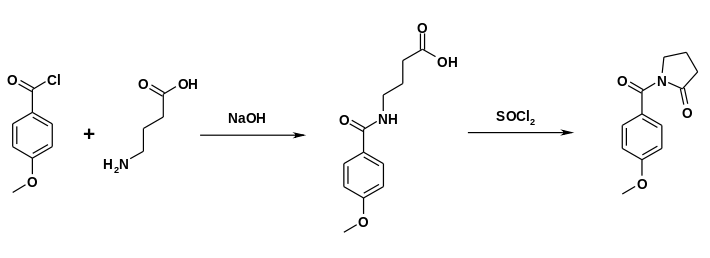
The drug was first made in the 1970s by Hoffmann-La Roche.[7][8] Synthesis can be accomplished by reacting 2-pyrrolidone with anisoyl chloride in the presence of triethylamine.[9]

Alternatively, gamma-aminobutyric acid can react with anisoyl chloride. Ring closure can be accomplished in the presence of thionyl chloride.[9]
Pharmacokinetics
When ingested orally aniracetam is quickly broken down via first pass hepatic metabolism. The primary metabolites of aniracetam are N-anisoyl-GABA, 2-pyrrolidone, and anisic acid.[10] Plasma concentrations are generally in the 5–15 μg/L range for aniracetam and 5–15 mg/L range for N-anisoyl-GABA, a pharmacologically-active metabolite, during the first few hours after oral administration of the drug. These two plasma species may be measured by liquid chromatography-mass spectrometry.[11][12][13]
See also
References
- ↑ Malykh AG; Sadaie MR (Feb 2010). "Piracetam and piracetam-like drugs: from basic science to novel clinical applications to CNS disorders.". Drugs. 70 (3): 287–312. doi:10.2165/11319230-000000000-00000. PMID 20166767.
- ↑ Ito; Tanabe, S; Kohda, A; Sugiyama, H; et al. (1990). "Allosteric potentiation of quisqualate receptors by a nootropic drug aniracetam". J. Physiol. 424: 533–543. PMC 1189827
 . PMID 1975272.
. PMID 1975272. - ↑ US 6730677, "Benzofurazan compounds which enhance AMPA receptor activity"
- ↑ Nakamura K; Kurasawa M (May 2001). "Anxiolytic effects of aniracetam in three different mouse models of anxiety and the underlying mechanism". Eur J Pharmacol. (Kanagawa, Japan). 420 (1): 33–43. doi:10.1016/S0014-2999(01)01005-6. PMID 11412837.
- ↑ Schizophrenia: New Insights for the Healthcare Professional: 2013 Edition. ScholarlyEditions. 22 July 2013. pp. 152–. ISBN 978-1-4816-6196-6.
- 1 2 Bernard Testa; Joachim M. Mayer (1 August 2003). Hydrolysis in Drug and Prodrug Metabolism. John Wiley & Sons. pp. 109–. ISBN 978-3-906390-25-3.
- ↑ Patent EP 5 143 Hoffmann-La Roche 1978
- ↑ Patent EP 44 088 Hoffmann-La Roche 1978
- 1 2 A. Kleemann, J. Engel, B. Kutscher, D. Reichert: Pharmaceutical Substances - Synthesis, Patents, Applications, 4. Auflage, Thieme 2001, ISBN 3-13-115134-X.
- ↑ Lee, CR; Benfield, P (1994). "Aniracetam. An overview of its pharmacodynamic and pharmacokinetic properties, and a review of its therapeutic potential in senile cognitive disorders". Drugs & aging. 4 (3): 257–73. doi:10.2165/00002512-199404030-00007. PMID 8199398.
- ↑ Cai S, Wang L. Determination of aniracetam's main metabolite, N-anisoyl-GABA, in human plasma by LC-MS/MS and its application to a pharmacokinetic study. J. Chromatogr. B 897: 50-54, 2012.
- ↑ Zhang J, Liang J, Tian Y, et al. Sensitive and selective liquid chromatography-tandem mass spectrometry method for the quantification of aniracetam in human plasma. J. Chromatogr. B 858: 129-134, 2007.
- ↑ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 10th edition, Biomedical Publications, Seal Beach, CA, 2014, p. 142-143.
