Lever rule
The lever rule is a tool used to determine weight percentages of each phase of a binary equilibrium phase diagram. It is used to determine the percent weight of liquid and solid phases for a given binary composition and temperature that is between the liquidus and solidus line. [1]
In an alloy with two phases, α and β, which themselves contain two elements, A and B, the lever rule states that the weight percentage of the α phase is
where
- a is the weight percentage of element B in the α phase
- b is the weight percentage of element B in the β phase
- c is the weight percentage of element B in the entire alloy
all at some fixed temperature.
Derivation
Suppose an alloy at an equilibrium temperature T consists of c wt% (weight percentage) of element B. Suppose also that at temperature T the alloy consists of two phases, α and β, for which the α consists of a wt% B, and β consists of b wt% B. Let the weight of the α phase in the alloy be so that the weight of the β phase is , where is the total weight of the alloy.
By definition, then, the total weight of element B in the α phase is , while the total weight of element B in the β phase is . Together these two quantities sum to the total weight of element B in the alloy, which is given by . Therefore
By rearranging, one finds that
This final quantity is the weight percentage of the α phase in the alloy.
Calculations
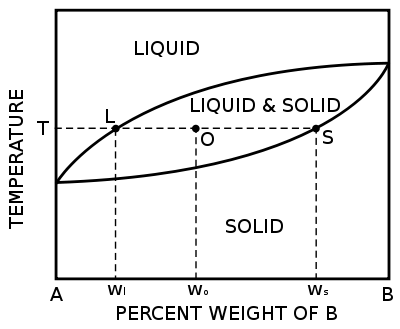
Binary phase diagrams
Before any calculations can be made, a tie line is drawn on the phase diagram to determine the percent weight of each element; on the phase diagram to the right it is line segment LS. This tie line is drawn horizontally at the composition's temperature from one phase to another (here the liquid to the solids). The percent weight of element B at the liquidus is given by wl and the percent weight of element B at the solidus is given by ws. The percent weight of solid and liquid can then be calculated using the following lever rule equations:[1]
where wo is the percent weight of element B for the given composition.
The numerator of each equation is the original composition we are interested in +/- the Opposite lever arm. That is if you want the percent weight of solid then take the difference between the liquid composition and the original composition. And then the denominator is the overall length of the arm so the difference between the solid and liquid compositions.
Eutectic phase diagrams

There is now more than one two-phase region. The tie line drawn is from the solid alpha to the liquid and by dropping a vertical line down at these points the wt% of each phase is directly read off the graph, that is the percentage weight of the x axis element. The same equations can be used to find the total percentage of alloy in each of the phases, i.e. Xs is the percentage of the whole sample in the liquid phase.[2]
References
- 1 2 Smith, William F.; Hashemi, Javad (2006), Foundations of Materials Science and Engineering (4th ed.), McGraw-Hill, pp. 318–320, ISBN 0-07-295358-6.
- ↑ Callister, William D.; Rethwisch, David (2009), Materials Science and Engineering An Introduction (8th ed.), Wiley, pp. 298–303, ISBN 978-0-470-41997-7.
