Velleral
 | |
| Names | |
|---|---|
| IUPAC name
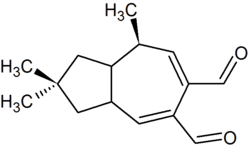
2,2,8-Trimethyl-1,2,3,3a,8,8a-hexahydro-5,6-azulenedicarbaldehyde | |
| Identifiers | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 20036851 |
| |
| |
| Properties | |
| C15H20O2 | |
| Molar mass | 232.146332 g/mol |
| Density | 1.093 g/cm3 |
| Boiling point | 341.125 °C (646.025 °F; 614.275 K) |
| Hazards | |
| Flash point | 127.95 °C (262.31 °F; 401.10 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Velleral (2,2,8-trimethyl-3,3a,8,8a-tetrahydro-1H-azulene-5,6-dicarbaldehyde) is a sesquiterpene dialdehyde found in certain mushrooms, like Lactarius torminosus[1] and Lactarius vellereus, after which it was named. The compound is thought to be part of a chemical defense system that protects the mushrooms against predation.[2] First isolated in 1969,[3] and characterized structurally in 1973,[4] velleral has antimicrobial activity.[5] Several syntheses have been devised.[6][7][8]
References
- ↑ Camazine S, Lupo TL Jr (1984). "Labile toxic compounds of the Lactarii: the role of the laticiferous hyphae as a storage depot for precursors of pungent dialdehydes". Mycologia. 76 (2): 355–8. doi:10.2307/3793113. JSTOR 3793113.
- ↑ Sterner O, Bergman R, Kihlberg J, Wickberg B (1985). "The sesquiterpenes of Lactarius vellereus and their role in a proposed chemical defense system". Journal of Natural Products. 48 (2): 279–88. doi:10.1021/np50038a013.
- ↑ List PH, Hackenberg H (1969). "Velleral und iso-Velleral, scharf schmeckende Stoffe aus Lactarius vellereus FRIES" [Velleral and iso-velleral, acrid-tasting compounds from Lactarius vellereus FRIES. 17. Mushroom contents]. Archiv der Pharmazie (in German). 302 (2): 125–43. doi:10.1002/ardp.19693020208. PMID 5260842.
- ↑ Magnusso G, Thoren S, Drakenbe T (1973). "Fungal extractives .4. Structure of a novel sesquiterpene dialdehyde from Lactarius by spectroscopic methods". Tetrahedron. 29 (11): 1621–4. doi:10.1016/S0040-4020(01)83407-4.
- ↑ Anke H, Sterner O (1991). "Comparison of the antimicrobial and cytotoxic activities of twenty unsaturated sesquiterpene dialdehydes from plants and mushrooms". Planta Medica. 57 (4): 344–6. doi:10.1055/s-2006-960114. PMID 1775575.
- ↑ Froborg J, Magnusson G, Thoren S (1975). "Fungal extractives .9. Synthesis of velleral skeleton and a total synthesis of pyrovellerolactone". Journal of Organic Chemistry. 40 (11): 1595–601. doi:10.1021/jo00899a017.
- ↑ Fex T, Froborg J, Magnusson G, Thoren S (1976). "Fungal extractives .10. Alternative synthesis of velleral skeleton". Journal of Organic Chemistry. 41 (22): 3518–20. doi:10.1021/jo00884a005.
- ↑ Froborg J, Magnusson G (1978). "Fungal extractives .12. Construction of vellerane skeleton with total syntheses of racemic velleral, vellerolactone, and pyrovellerolactone – revised structures". Journal of the American Chemical Society. 100 (21): 6728–33. doi:10.1021/ja00489a030.
This article is issued from Wikipedia - version of the 6/6/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.
