Ammonium iron(III) sulfate
 | |
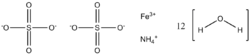 | |
| Names | |
|---|---|
| IUPAC name
Ammonium iron(III) sulfate | |
| Other names
Ferric ammonium sulfate Ferric alum | |
| Identifiers | |
| 10138-04-2 7783-83-7 (dodecahydrate) | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 55405 |
| ECHA InfoCard | 100.107.412 |
| UNII | LUX2X1H1IC |
| |
| |
| Properties | |
| FeNH4(SO4)2 | |
| Molar mass | 482.25 g/mol (dodecahydrate) |
| Appearance | Pale violet octahedral crystals |
| Odor | weak ammonia-like |
| Density | 1.71 g/cm3 |
| Melting point | 39 to 41 °C (102 to 106 °F; 312 to 314 K) |
| 1240 g/L | |
| Hazards | |
| Main hazards | Irritant (Xi) |
| Related compounds | |
| Other anions |
Ammonium iron(III) citrate Ammonium chloride |
| Other cations |
Ammonium aluminium sulfate potassium aluminium sulfate |
| Related compounds |
Ammonium iron(II) sulfate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Ammonium iron(III) sulfate, NH4Fe(SO4)2·12 H2O, or NH4[Fe(H2O)6](SO4)2·6 H2O, also known as ferric ammonium sulfate (FAS) or iron alum, is a double salt in the class of alums, which consists of compounds with the general formula AB(SO4)2 · 12 H2O.[1] It has the appearance of weakly violet, octahedrical crystals. There has been some discussion regarding the origin of the crystals' colour, with some ascribing it to impurities in the compound,[2] and others claiming it to be a property of the crystal itself.[3]
FAS is paramagnetic,[4] acidic and toxic towards microorganisms.[5] It is a weak oxidizing agent, capable of being reduced to Mohr's salt, ferrous ammonium sulfate.
Preparation
FAS can be prepared by crystallization from a solution of ferric sulfate and ammonium sulfate. Iron(II) in ferrous sulfate is oxidized to Iron(III) in ferric sulfate by addition of sulfuric and nitric acid. Upon addition of ammonium sulfate to the solution and damping in of the solution, ferric ammonium sulfate crystals will precipitate.
Oxidation: 6 FeSO4 + 2 HNO3 + 3 H2SO4 = 3 Fe2(SO4)3 + 2 NO + 4 H2O
Synthesis: Fe2(SO4)3 + (NH4)2SO4 = 2 NH4Fe(SO4)2
Procedure[6]:
The nitric and sulfuric acid is reacted with the ferrous sulfate to make ferric sulfate, nitric oxide, and water. The ferric sulfate is mixed with ammonium sulfate and crystallized to get ammonium iron(III) sulfate. The solution is normally tested to ensure that no more iron(II) is left.

Uses
Areas of use for FAS include waste water treatment,[7] tanning,[7] production of dyestuffs,[7] and as an etching agent in the production of electronic components.[8] It has been used in a wide area of applications, including adiabatic refrigeration equipment,[9] biochemical analysis[10] and organic synthesis.[11]
References
- ↑ Considine, Douglas M: Chemical and process technology encyclopedia, McGraw-Hill, New York, 1974, p. 993
- ↑ Christensen, Odin T. "On the Cause of the Amethyst Color of Ferric Alum and of Mixed Crystals of Ferric and Manganic Alum". Chem. Lab. Roy. Vet. Agr. Hochschule, Kgl. Danske Vidsk. Selsk. Forh. 1906: 173–95.
- ↑ Bonnell, Jane; Philip Perman, Edgar (1921). "CCXXIX.—The colour of iron alum". J. Chem. Soc., Trans. 119: 1994–1997. doi:10.1039/CT9211901994.
- ↑ Cooke, Meyer; Wolf. "The Specific Heats of Three Paramagnetic salts at Very Low Temperatures". Proceedings of the Royal Society of London. Series A, Mathematical and Physical Sciences. 237 (1210): 395–403. doi:10.1098/rspa.1956.0185.
- ↑ Wang, Fei; et al. "Microcalorimetric investigation of the toxic action of ammonium ferric(III)sulfate on the metabolic activity of pure microbes". Environmental Toxicology and Pharmacology. 25: 351–357. doi:10.1016/j.etap.2007.11.004.
- ↑ Hecht, Horstmar: Prãparative Anorganische Chemie, Springer-Verlag, Berlin, 1951. p. 127
- 1 2 3 Wiley Encyclopedia of inorganic chemistry: Volume 4, p. 1704:
- ↑ Chen et al.: United States Patent 5518131 - "Etching molydbenum with ferric sulfate and ferric ammonium sulfate"
- ↑ Grant W. Wilson, Peter T. Timbie: Construction techniques for adiabatic demagnetization refrigerators using ferric ammonium alum. Cryogenics, *Volume 39, Number 4, (1999) , p. 319–322
- ↑ J. C. Whitehorn: A system of blood analysis. Supplement II. Simplified method for the determination of chlorides in blood or plasma. *Journal of Biological Chemistry (1921), 45 p. 449–60.
- ↑ Yu, Shanxin; et al. (2005). "Application of ammonium ferric sulfate dodecahydrate in organic synthesis". General Review. 17 (1): 27–30.
| Salts and esters of the sulfate ion | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2SO4 | He | ||||||||||||||||||
| Li2SO4 | BeSO4 | B | esters ROSO3− (RO)2SO2 |
(NH4)2SO4 N2H6SO4 (NH3OH)2SO4 |
O | F | Ne | ||||||||||||
| Na2SO4 NaHSO4 |
MgSO4 | Al2(SO4)3 Al2SO4(OAc)4 |
Si | P | SO42− | Cl | Ar | ||||||||||||
| K2SO4 KHSO4 |
CaSO4 | Sc2(SO4)3 | Ti(SO4)2 TiOSO4 |
V2(SO4)3 VOSO4 |
CrSO4 Cr2(SO4)3 |
MnSO4 | FeSO4 Fe2(SO4)3 |
CoSO4, Co2(SO4)3 |
NiSO4 | CuSO4 | ZnSO4 | Ga2(SO4)3 | Ge | As | Se | Br | Kr | ||
| RbHSO4 Rb2SO4 |
SrSO4 | Y2(SO4)3 | Zr(SO4)2 | Nb | Mo | Tc | Ru | Rh | PdSO4 | Ag2SO4 | CdSO4 | In2(SO4)3 | SnSO4 | Sb2(SO4)3 | Te | I | Xe | ||
| Cs2SO4 | BaSO4 | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg2SO4, HgSO4 |
Tl2SO4 | PbSO4 | Bi2(SO4)3 | Po | At | Rn | |||
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La | Ce2(SO4)3 Ce(SO4)2 |
Pr2(SO4)3 | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb2(SO4)3 | Lu | |||||
| Ac | Th | Pa | U(SO4)2 UO2SO4 |
Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||
