Capecitabine
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /kæpᵻˈsaɪtəbin/ |
| Trade names | Xeloda |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a699003 |
| Pregnancy category | |
| Routes of administration | Oral |
| ATC code | L01BC06 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Extensive |
| Protein binding | < 60% |
| Metabolism | Hepatic, to 5'-DFCR, 5'-DFUR (inactive); neoplastic tissue, 5'-DFUR to active fluorouracil |
| Biological half-life | 38–45 minutes |
| Excretion | Renal (95.5%), faecal (2.6%) |
| Identifiers | |
| |
| CAS Number |
154361-50-9 |
| PubChem (CID) | 60953 |
| IUPHAR/BPS | 6799 |
| DrugBank |
DB01101 |
| ChemSpider |
54916 |
| UNII |
6804DJ8Z9U |
| KEGG |
D01223 |
| ChEBI |
CHEBI:31348 |
| ChEMBL |
CHEMBL1773 |
| ECHA InfoCard | 100.112.980 |
| Chemical and physical data | |
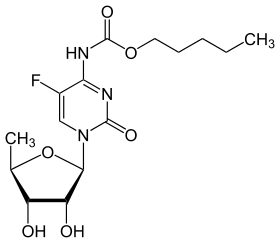
| Formula | C15H22FN3O6 |
| Molar mass | 359.35 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Capecitabine (INN) is an orally-administered chemotherapeutic agent used in the treatment of numerous cancers.[1] Capecitabine is a prodrug that is enzymatically converted to 5-fluorouracil (5-FU) in the body.[2]
It is on the World Health Organization's List of Essential Medicines, a list of the most important medications needed in a basic health system.[3]
Medical uses
It is used in the treatment of the following cancers:[1][2][4]
- Colorectal cancer (either as neoadjuvant therapy with radiation, adjuvant therapy or for metastatic cases)
- Breast cancer (metastatic or as monotherapy/combotherapy; this is licensed as a second-line treatment in the UK)
- Gastric cancer (off-label in the US; this is a licensed indication in the UK)
- Oesophageal cancer (off-label in the US)
It is often referred to as Xeloda, the name under which it is marketed by Genentech. It is available in 500-mg and 150-mg tablets.
Adverse effects
Adverse effects by frequency:[5][6][7][8]
- Very common (>10% frequency)
- Appetite loss
- Diarrhea
- Vomiting
- Nausea
- Stomatitis
- Abdominal pain
- Fatigue
- Weakness
- Hand-foot syndrome[9]
- Oedema
- Fever
- Pain
- Headache
- Hair loss
- Dermatitis
- Indigestion
- Shortness of breath
- Eye irritation
- Myelosuppression[Note 1]
Notes on adverse effects:
- ↑ Includes: anaemia, lymphopenia, neutropenia and thrombocytopenia
Contraindications
Contraindications include:[7]
- History of hypersensitivity to fluorouacil, capecitabine or any of its excipients
- DPD deficiency (see Pharmacogenetics)
- Pregnancy and lactation
- Severe leucopenia, neutropenia, or thrombocytopenia
- Severe hepatic impairment or severe renal impairment
- Treatment with sorivudine or its chemically related analogues, such as brivudine
Drug interactions
Drugs it is known to interact with include:[7]
- Sorivudine or its analogues, such as, brivudine.
- Allopurinol as it decreases the efficacy of 5-FU.
- CYP2C9 substrates, including, warfarin and other coumarin-derivatives anticoagulants
- Phenytoin, as it increases the plasma concentrations of phenytoin.
- Calcium folinate may enhance the therapeutic effects of capecitabine by means of synergising with its metabolite, 5-FU. It may also induce more severe diarrhoea by means of this synergy.[1]
Pharmacogenetics
The dihydropyrimidine dehydrogenase (DPD) enzyme is responsible for the detoxifying metabolism of fluoropyrimidines, a class of drugs that includes capecitabine, 5-fluorouracil and tegafur.[10] Genetic variations within the DPD gene (DPYD) can lead to reduced or absent DPD activity, and individuals who are heterozygous or homozygous for these variations may have partial or complete DPD deficiency; an estimated 0.2% of individuals have complete DPD deficiency.[10][11] Those with partial or complete DPD deficiency have a significantly increased risk of severe or even fatal drug toxicities when treated with fluoropyrimidines; examples of toxicities include myelosuppression, neurotoxicity and hand-foot syndrome.[10][11]
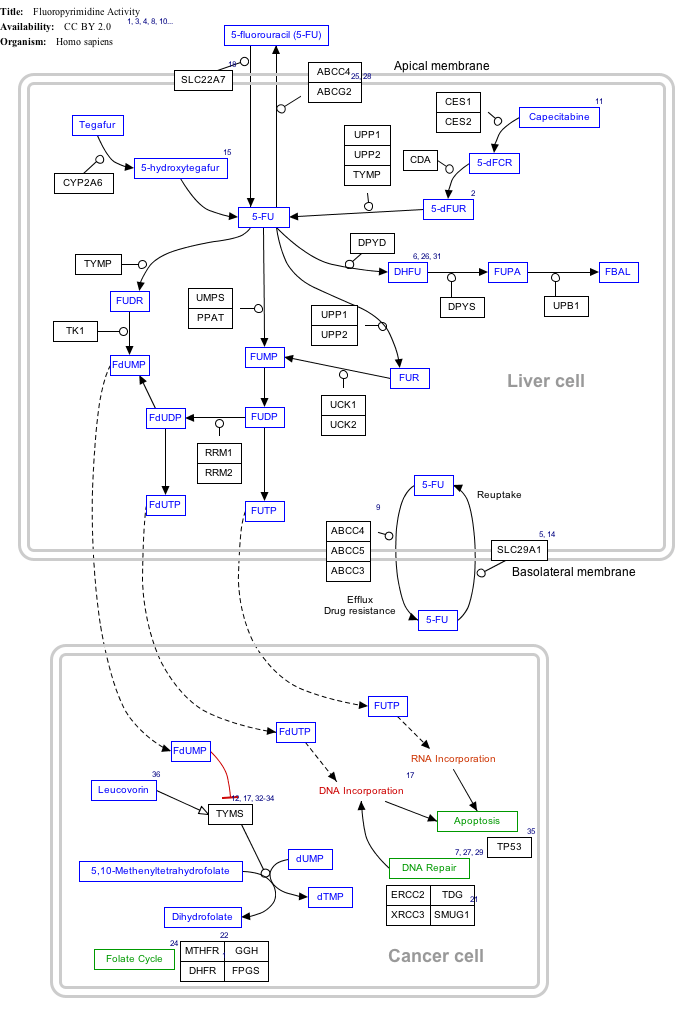
Mechanism of action
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
Fluorouracil (5-FU) Activity edit
- ↑ The interactive pathway map can be edited at WikiPathways: "FluoropyrimidineActivity_WP1601".
Capecitabine is metabolised to 5-FU which in turn is a thymidylate synthase inhibitor, hence inhibiting the synthesis of thymidine monophosphate (ThMP), the active form of thymidine which is required for the de novo synthesis of DNA.[2]
References
- 1 2 3 Rossi, S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- 1 2 3 "Xeloda (capecitabine) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. 25 January 2014.
- ↑ "www.who.int" (PDF).
- ↑ Joint Formulary Committee (2013). British National Formulary (BNF) (65 ed.). London, UK: Pharmaceutical Press. ISBN 978-0-85711-084-8.
- ↑ "XELODA (capecitabine) tablet, film coated [Genentech, Inc.]". DailyMed. Genentech, Inc. December 2013. Retrieved 25 January 2014.
- ↑ "Capecitabine Teva : EPAR - Product Information" (PDF). European Medicines Agency. Teva Pharma B.V. 10 January 2014. Retrieved 25 January 2014.
- 1 2 3 "Capecitabine 150mg - Summary of Product Characteristics (SPC)". electronic Medicines Compendium. Zentiva. 23 December 2013. Retrieved 25 January 2014.
- ↑ "NAME OF THE MEDICINE XELODA® Capecitabine" (PDF). TGA eBusiness Services. Roche Products Pty Limited. 5 December 2013. Retrieved 25 January 2014.
- ↑ Reddening, swelling, numbness and desquamation on palms and soles
- 1 2 3 Caudle, KE; Thorn, CF; Klein, TE; Swen, JJ; McLeod, HL; Diasio, RB; Schwab, M (December 2013). "Clinical Pharmacogenetics Implementation Consortium guidelines for dihydropyrimidine dehydrogenase genotype and fluoropyrimidine dosing.". Clinical pharmacology and therapeutics. 94 (6): 640–5. doi:10.1038/clpt.2013.172. PMID 23988873.
- 1 2 Amstutz, U; Froehlich, TK; Largiadèr, CR (September 2011). "Dihydropyrimidine dehydrogenase gene as a major predictor of severe 5-fluorouracil toxicity.". Pharmacogenomics. 12 (9): 1321–36. doi:10.2217/pgs.11.72. PMID 21919607.
External links
- Xeloda.com (patient information, tools, and resources)
- OralChemo Advisor (patient information)