Cladribine
 | |
| Clinical data | |
|---|---|
| Trade names | Leustatin, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a693015 |
| Pregnancy category | |
| Routes of administration | Intravenous, subcutaneous (liquid) |
| ATC code | L01BB04 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 100% (i.v.) |
| Protein binding | 25% (range 5-50%)[2] |
| Metabolism | Mostly via intracellular kinases; 15-18% is excreted unchanged[2] |
| Biological half-life | Terminal elimination half-life: Approximately 10 hours after both intravenous infusion an subcutaneous bolus injection[2] |
| Excretion | Urinary[2] |
| Identifiers | |
| |
| CAS Number |
4291-63-8 |
| PubChem (CID) | 20279 |
| IUPHAR/BPS | 4799 |
| DrugBank |
DB00242 |
| ChemSpider |
19105 |
| UNII |
47M74X9YT5 |
| KEGG |
D01370 |
| ChEBI |
CHEBI:567361 |
| ChEMBL |
CHEMBL1619 |
| ECHA InfoCard | 100.164.726 |
| Chemical and physical data | |
| Formula | C10H12ClN5O3 |
| Molar mass | 285.687 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
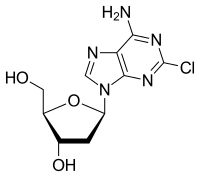
Cladribine is a medication used to treat hairy cell leukemia (HCL, leukemic reticuloendotheliosis) and B-cell chronic lymphocytic leukemia.[3][4] Its chemical name is 2-chloro-2'-deoxyadenosine (2CdA).
As a purine analog, it is a synthetic chemotherapy agent that targets lymphocytes and selectively suppresses the immune system. Chemically, it mimics the nucleoside adenosine and thus inhibits the enzyme adenosine deaminase, which interferes with the cell's ability to process DNA. Cladribine is activated only by lymphocytes, and non-activated cladribine is removed quickly from all other cells. This means that there is very little non-target cell loss.[3][5]
Medical uses
Cladribine is used for as a first and second-line treatment for symptomatic hairy cell leukemia and for B-cell chronic lymphocytic leukemia and is administered by intravenous infusion.[4][6]
It used, often in combination with other cytotoxic agents, to treat various kinds of histiocytosis, including Erdheim–Chester disease[7] and Langerhans cell histiocytosis,[8]
Clabridine can cause fetal harm when administered to a pregnant woman and is listed by the FDA as Pregnancy Category D; safety and efficacy in children has not been established.[6]
Adverse effects
Injectable cladribine suppresses the body's ability to make new blood cells (called Myelosuppression); data from HCL studies showed that about 70% of people taking the drug had fewer white blood cells and about 30% developed infections and some of those progressed to septic shock; about 40% of people taking the drug had fewer red blood cells and became severely anemic; and about 10% of people had too few platelets.[6]
At the dosage used to treat HCL in two clinical trials, 16% of people had rashes and 22% had nausea, the nausea generally did not lead to vomiting.[6]
History
Ernest Beutler and Dennis A. Carson had studied adenosine deaminase deficiency and recognized that because the lack of adenosine deaminase led to the destruction of B cell lymphocytes, a drug designed to inhibit adenosine deaminase might be useful in lymphomas. Carson then synthesized cladribine, and through clinical research at Scripps starting in the 1980s, Beutler tested it as intravenous infusion and found it was especially useful to treat hairy cell leukemia (HCL). No pharmaceutical companies were interested in selling the drug because HCL was an orphan disease, so Beutler's lab synthesized and packaged it and supplied it to the hospital pharmacy; the lab also developed a test to monitor blood levels. This was the first treatment that led to prolonged remission of HCL, which was previously untreatable.[9]:14-15
In February 1991 Scripps began a collaboration with Johnson & Johnson to bring intravenous cladribine to market and by December of that year J&J had filed an NDA; cladrabine was approved by the FDA in 1993 for HCL as an orphan drug,[10] and was approved in Europe later that year.[11]:2
The subcutaneous formulation was developed in Switzerland in the early 1990s and it was commercialized by Lipomed GmbH in the 2000s.[11]:2[12]
In the mid-1990s Buetler, in collaboration with Jack Sipe, a neurologist at Scripps, ran several clinical trials exploring the utility of cladribine in multiple sclerosis, based on the drug's immunosuppressive effects, Sipe's insight into MS, and Buetler's interest in MS due to his sister's having it.[9]:17[13] Ortho-Clinical, a subsidiary of J&J, filed an NDA for cladribine for MS in 1997 but withdrew it in the late 1990s after discussion with the FDA proved that more clinical data would be needed.[14][15]
Ivax acquired the rights for oral administration of cladribine to treat MS from Scripps in 2000,[16] and partnered with Serono in 2002.[15] Ivax was acquired by Teva in 2006,[17][18] and Merck KGaA acquired control of Serono's drug business in 2006.[19]
An oral formulation of the drug with cyclodextrin was developed[20]:16 and Ivax and Serono, and then Merck KGgA conducted several clinical studies. Merck KGgA submitted an application to the European Medicines Agency in 2009, which was rejected in 2010, and an appeal was denied in 2011.[20]:4-5 Likewise Merck KGgA's NDA with the FDA rejected in 2011.[21] The concerns were that several cases of cancer had arisen, and the ratio of benefit to harm was not clear to regulators.[20]:54-55 The failures with the FDA and the EMA were a blow to Merck KGgA and were one of a series of events that led to a reorganization, layoffs, and closing the Swiss facility where Serono had arisen.[22][23] However, several MS clinical trials were still ongoing at the time of the rejections, and Merck KGgA committed to completing them.[21] A meta-analysis of data from clinical trials showed that cladiribine did not increase the risk of cancer at the doses used in the clinical trials.[24] In 2015 Merck KGgA announced it would again seek regulatory approval with data from the completed clinical trials in hand,[22] and in 2016 the EMA accepted its application for review.[25]
Research directions
Cladribine has been studied as part of a multi-drug chemotherapy regimen for drug-resistant T-cell prolymphocytic leukemia.[26]
References
- ↑ Drugs.com International trade names for Cladribine Page accessed Jan 14, 2015
- 1 2 3 4 "PRODUCT INFORMATION LITAK© 2 mg/mL solution for injection" (PDF). TGA eBusiness Services. St Leonards, Australia: Orphan Australia Pty. Ltd. 10 May 2010. Retrieved 27 November 2014.
- 1 2 "European Medicines Agency - - Litak". www.ema.europa.eu.
- 1 2 "Leustat Injection. - Summary of Product Characteristics (SPC) - (eMC)". www.medicines.org.uk.
- ↑ Leist, TP; Weissert, R (2010). "Cladribine: mode of action and implications for treatment of multiple sclerosis.". Clinical neuropharmacology. 34 (1): 28–35. PMID 21242742.
- 1 2 3 4 Cladribine label, last updated July 2012. Page accessed January 14, 2015
- ↑ Histiocytosis Association Erdheim-Chester Disease Page accessed Aug 20, 2016
- ↑ Aricò M. Langerhans cell histiocytosis in children: from the bench to bedside for an updated therapy. Br J Haematol. 2016 Jun;173(5):663-70. Review. PMID 26913480. Quote: "The combination of cytarabine and cladribine is the current standard for second-line therapy of refractory cases with vital organ dysfunction."
- 1 2 Marshall A. Lichtman Biographical Memoir: Ernest Beutler 1928–2008 National Academy of Sciences, 2012
- ↑ Staff, The Pink Sheet Mar 8, 1993 Ortho Biotech’s Leustatin For Hairy Cell Leukemia
- 1 2 EMA 2004 Litak EMA package: Scientific Discussion
- ↑ EMA 2004 Litak: Background Information one the Procedure
- ↑ Eric Sauter and Mika Ono for Scripps News and Views. Vol 9. Issue 18. June 1, 2009 A Potential New MS Treatment's Long and Winding Road
- ↑ Tortorella C, et al. Cladribine. Ortho Biotech Inc. Curr Opin Investig Drugs. 2001 Dec;2(12):1751-6. PMID 11892941
- 1 2 Carey Sargent for Dow Jones Newswires in the Wall Street Journal. Oct. 31, 2002 Serono Purchases Rights To Experimental MS Drug
- ↑ Reuters. Dec 4, 2000. Ivax to Develop Cladribine for Multiple Sclerosis
- ↑ Jennifer Bayot for the New York Times. July 26, 2005 Teva to Acquire Ivax, Another Maker of Generic Drugs
- ↑ Teva Press Release, 2006. Teva Completes Acquisition of Ivax
- ↑ Staff, First Word Pharma. Sept 21, 2006 http://www.firstwordpharma.com/node/135767#axzz4HwfDP98J Merck KGaA to acquire Serono]
- 1 2 3 EMA. 2011 Withdrawal Assessment Report for Movectro Procedure No. EMEA/H/C/001197
- 1 2 John Gever for MedPage Today June 22, 2011 06.22.2011 0 Merck KGaA Throws in Towel on Cladribine for MS
- 1 2 John Carroll for FierceBiotech Sep 11, 2015 Four years after a transatlantic slapdown, Merck KGaA will once again seek cladribine OK
- ↑ Connolly, Allison (24 April 2012). "Merck KGaA to Close Merck Serono Site in Geneva, Cut Jobs". Bloomberg.
- ↑ Pakpoor, J; et al. (December 2015). "No evidence for higher risk of cancer in patients with multiple sclerosis taking cladribine.". Neurology(R) neuroimmunology & neuroinflammation. 2 (6): e158. PMC 4592538
 . PMID 26468472.
. PMID 26468472. - ↑ Press release:
- ↑ Hasanali, Zainul S.; Saroya, Bikramajit Singh; Stuart, August; Shimko, Sara; Evans, Juanita; Shah, Mithun Vinod; Sharma, Kamal; Leshchenko, Violetta V.; Parekh, Samir (24 June 2015). "Epigenetic therapy overcomes treatment resistance in T cell prolymphocytic leukemia". Science Translational Medicine. 7 (293): 293ra102–293ra102. doi:10.1126/scitranslmed.aaa5079. ISSN 1946-6234. PMID 26109102.