Dacomitinib
 | |
| Names | |
|---|---|
| IUPAC name
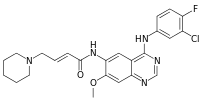
(2E)-N-{4-[(3-Chloro-4-fluorophenyl)amino]-7-methoxy-6-quinazolinyl}-4-(1-piperidinyl)-2-butenamide | |
| Other names
PF-00299804 | |
| Identifiers | |
| 1110813-31-4 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:132268 |
| ChEMBL | ChEMBL2105719 |
| ChemSpider | 9685914 |
| 7422 | |
| PubChem | 11511120 |
| UNII | 2XJX250C20 |
| |
| |
| Properties | |
| C24H25ClFN5O2 | |
| Molar mass | 469.95 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dacomitinib (PF-00299804) is an experimental drug candidate under development by Pfizer for the treatment of non-small-cell lung carcinoma. It is a selective and irreversible inhibitor of EGFR.[1]
Dacomitinib has advanced to several Phase III clinical trials. The results of the first trials were disappointing, with a failure to meet the study goals,[2][3][4] Additional Phase III trials are ongoing.[2]
References
- ↑ "Dacomitinib". NCI Drug Dictionary.
- 1 2 Zosia Chustecka (January 27, 2014). "Dacomitinib Fails in Pretreated Non-small Cell Lung Cancer". Medscape.
- ↑ "Blow to Pfizer as dacomitinib fails in lung cancer trials". pmlive.com. 28 January 2014.
- ↑ "Pfizer Announces Top-Line Results From Two Phase 3 Trials Of Dacomitinib In Patients With Refractory Advanced Non-Small Cell Lung Cancer". Pfizer Press Release. January 27, 2014.
External links
- Dacomitinib Fact Sheet, Pfizer
This article is issued from Wikipedia - version of the 9/26/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.
