Leishmania donovani
| Leishmania donovani | |
|---|---|
 | |
| Leishmania donovani in bone marrow cell | |
| Scientific classification | |
| Domain: | Eukaryota |
| (unranked): | Excavata |
| Phylum: | Euglenozoa |
| Class: | Kinetoplastida |
| Order: | Trypanosomatida |
| Family: | Trypanosomatidae |
| Genus: | Leishmania |
| Species: | Leishmania donovani |
| Binomial name | |
| Leishmania donovani (Laveran et Mesnil, 1903) Ross, 1903 | |
Leishmania donovani is a species of intracellular parasitic protozoan belonging to the genus Leishmania, a group of haemoflagellate kinetoplastids that cause the disease leishmaniasis. It is a human blood parasite responsible for visceral leishmaniasis or kala-azar, the most severe form of leishmaniasis. It infects the mononuclear phagocyte system including spleen, liver and bone marrow. Infection is transmitted by species of sandfly belonging to the genus Phlebotomus in Old World and Lutzomyia in New World. Therefore, the parasite is prevalent throughout tropical and temperate regions including Africa (mostly in Sudan), China, India, Nepal, southern Europe, Russia and South America.[1][2][3] It is responsible for thousands of deaths every year and has spread to 88 countries, with 350 million people at constant risk of infection and 0.5 million new cases in a year.[4]
L. donovani was independently discovered by two British medical officers William Boog Leishman in Netley, England, and Charles Donovan in Madras, India, in 1903. However, the correct taxonomy was provided by Ronald Ross. The parasite requires two different hosts for a complete life cycle, humans as the definitive host and sandflies as the intermediate host. In some parts of the world other mammals, especially canines, act as reservoir hosts. In human cell they exist as small, spherical and unflagellated amastigote form; while they are elongated with flagellum as promastigote form in sandflies. Unlike other parasitic protists they are unable to directly penetrate the host cell, and are dependent upon phagocytosis.[5][6] The whole genome sequence of L. donovani obtained from southeastern Nepal was published in 2011.[7]
Discovery
One of the earliest known epidemics of L. donovani infection (kala-azar as it was called in Hindi) was known in India just after the Indian Rebellion of 1857. The first medical record was however only in 1870 by British medical officers from Assam. In 1900, an English soldier stationed at Dum Dum, West Bengal, died at the Army Medical School in Netley, England. The autopsy was performed by William Boog Leishman. He processed the tissue sample of the enlarged spleen using a staining technique (now known as Leishman's stain) which he had just developed, and discovered the protozoan parasites using microscopy. But he mistakenly considered the parasites to be degenerate trypanosomes, already known protozoan parasites in Africa and South America. In 1903, Leishman published his discovery of "trypanosomes in India" in the British Medical Journal, which appeared on 11 May. Another British medical officer Charles Donovan, who was serving in the Indian Medical Service, had found the parasites in April of that year at the Government General Hospital in Madras. After reading Leishman paper, Donovan confirmed on 17 June that the parasites (by then known as "Leishman bodies") were definitely the causative agents of kala-azar. He wrote a commentary of his discovery in relation to that of Leishman in the same journal, that appeared on 11 July 1903.[8] Soon a controversy arose as to whom such a monumental discovery should be credited. Donovan sent some of his slides to Ronald Ross, who was at the time in Liverpool, and to Alphonse Laveran at the Pasteur Institute in Paris. Laveran and his colleague Félix Mesnil identified the protozoan (and yet wrongly) to be members of Piroplasmida, and gave the scientific name Piroplasma donovanii. It was Ross who resolved the conflict of priority in the discovery and correctly identified the species as member of the novel genus Leishmania. He gave the popular name "Leishman-Donovan bodies", and subsequently the valid binomial Leishmania donovani, thereby equally crediting the two rivals.[9][10][11][12]
Structure
Leishmania donovani is a unicellular eukaryote having a well-defined nucleus and other cell organelles including a kinetoplast and a flagellum. Depending on its host it exists in two structural variants, as follows:[12][13][14][15]
- Amastigote form found in the mononuclear phagocyte and circulatory systems of humans. It is an intracellular and non-motile form, being devoid of external flagellum. The short flagellum is embedded in the anterior end without projecting out. It is oval in shape, and measures 3–6 µm in length and 1–3 µm in breadth. The kinetoplast and basal body lie towards the anterior end.
- Promastigote is formed in the alimentary tract of the sandfly. It is an extracellular and motile form. It is considerably larger and more highly elongated, measuring 15–30 µm in length and 5 µm in width. It is spindle-shaped, tapering at both ends. A long flagellum (about the body length) is projected externally at the anterior end. The nucleus lies at the centre, and in front of which are kinetoplast and basal body.
Infection and life cycle
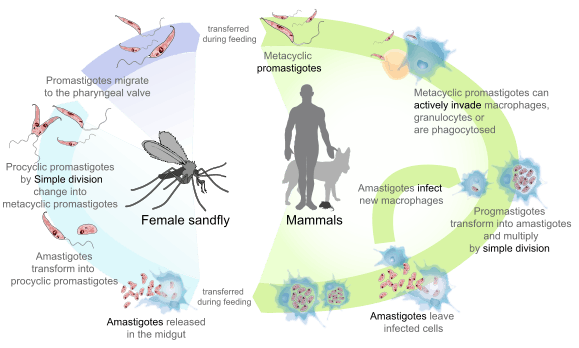
Leishmania donovani is a digenetic parasite passing its life cycle in two different hosts.
Definitive host
In humans the metacyclic promastigotes are injected by sandfly through the skin during its blood meal. When sandfly bites using its proboscis it ejects the parasites that are stored inside the hollow tube. Some promastigotes may enter the blood stream directly where some are destroyed by macrophagic cytolysis. But many are also taken up through phagocytosis by mononuclear phagocytes in liver, spleen and bone marrow.[16] Inside the cells they undergo spontaneous transformation into oval-shaped amastigotes.[17][18] Granulocytes selectively kill the promastigotes by oxidative mechanism, while amastigotes are resistant.[19] Then the surviving amastigotes undergo cell division using simple binary fission. Multiplication continues until the host cell can no longer hold and ruptures. In a fully congested cell there can be as many as 50 to 200 amastigotes, which are released into tissue cavities. Each individual amastigote is then capable of invading fresh cells. As a result, the entire tissue is progressively infected and destroyed. A number of free amastigotes then enters the blood stream where many are phagocytosed by macrophages. These free and phagocytosed amastigotes in peripheral blood are then sucked up by blood-feeding sandfly.[20][21][22]
Intermediate host
L. donovani undergo further development only in the digestive tract of the female sandfly. Hence only females are responsible for transmitting the infection. Once the amastigotes are ingested, they enter the midgut of the sandfly. Then they undergo structural modification into flagellated promastigotes, becoming larger and considerably elongated. They get attached to the gut epithelial lining where they multiply rapidly by binary fission. (They are also capable of sexual reproduction by genetic hybridisation in the sandfly gut.)[23] They then migrate back towards the anterior part of the digestive system such as pharynx and buccal cavity. This process is known as anterior station development, which is unique in Leishmania. A heavy infection of pharynx can be observed within 6 to 9 days after initial blood meal. The promastigotes become infective only by this time, and the event is called the metacyclic stage.[12][21][24] The metacyclic promastigotes then enter the hollow proboscis where they accumulate and completely block the food passage. Immediately upon biting a human, the parasites are released, which invariably results in infection. The stages of development in sandfly can be described as follows:[13]
- Soon after entering the gut, the amastigotes get coated with peritrophic matrix, which is composed of chitin and protein complex. This protects the parasites from the digestive enzymes of the host.
- The amastigotes travel as far as the abdominal midgut and first transform into a weakly motile "procyclic promastigotes" on the gut wall within 1–3 days.
- The young promastigotes secrete a neuropeptide that stop peristalsis of the gut. The surface lipophosphoglycan (LPG) of the promastigote serves as an attachment to the gut epithelium. These factors prevent the expulsion of promastigotes during excretion of the insect.[25]
- During 4–7 days the peritrophic matrix is degraded by the activity of chitinases. This release the more actively motile "nectomonad promastigotes" which migrate anteriorly until they reach the opening of the thoracic gut.
- Another transformation takes place by which they turn into "leptomonad promastigotes". These are fully motile and capable of binary fission. Multiplication and migration towards thoracic midgut cause congestion of the pharynx and buccal cavity. Here they secrete promastigote secretory gel (PSG), which is composed of soluble acid phosphatase and phosphoglycoprotein.[26][27]
- After 6–9 days the promastigotes become metacyclic. Some are also transformed into non-replicating promastigotes, which also become metacyclic. The sandfly is able to regurgitate and eject the parasites from its proboscis with the help of PSG when it bites.
Reservoir host
Dogs are known to be susceptible to L. donovani infection.[28] Especially in the New World, infection is a zoonotic disease, involving different canine species, including domestic dog and the two fox species, Lycalopex vetulus and Cerdocyon thous. In the Mediterranean region domestic dogs and the three fox species Vulpes vulpes, V. corsac and V. zerda are common reservoir hosts.[29][30] In Africa and Brazil, some marsupials and rodents are also reported to harbour L. donovani.[31]
Epidemiology
It is estimated that visceral leishmaniasis (VL) affects more than 100 million people worldwide, with 500,000 new cases and more than 50,000 deaths each year.[4][32] Although L. donovani is only the second-most prevalent Leishmania causing VL, it is the most dangerous form and directly fatal to humans. Over 90% of reported cases are from India, Bangladesh, Nepal, Sudan and Brazil.[33] In India it is prevalent in the eastern region including Bihar, West Bengal, eastern Uttar Pradesh, Assam and foothills of Sikkim.[34] It is responsible for tens of thousands of mortality among Africans in eastern and southern parts of Sudan. During the epidemic of 1984–1994 death toll was as high as 70% in the Sudanese population.[35] Moreover, due to emergence of drug resistance the prevalence is not subsiding, and in fact has spread to central Europe. For example, during the late 1990s hundreds of cases were reported in Switzerland.[36]
Pathogenicity
L. donovani is the causative agent of visceral leishmaniasis, traditionally known as kala-azar ("black fever", particularly in India), because of its characteristic symptoms. The disease is highly lethal if not treated properly. The incubation period generally ranges from 3 to 6 months, and in some cases may be over a year. In Indian leishmaniasis, incubation can be as short as 10 days. The target cells are those of mononuclear phagocyte system. The two main tissues of infection are spleen and liver.[37] Clinical symptoms include pyrexia (recurring high fever which may be continuous or remittent), enlargement of spleen and liver, and heavy skin pigmentation which darkens the physical appearance (the reason for naming "black fever"). To a lesser extent mucosa of the small intestine and lymph nodes are also invaded by the parasite. Morphological symptoms are noticeable particularly on facial and abdominal regions. Skin becomes coarse and hard. In African infections, warty eruptions are common. In a fully developed stage, the patient shows emaciation and anaemia. Where medical facilities are poor, mortality can be as high as 75–95% within 2 years of epidemics. The disease is often accompanied by complications with dysentery, tuberculosis, septicaemia and even HIV infection.[21][38][39]
Cellular invasion and immunological response
Amastigotes of L. donovani enter macrophages via a Rac1- and Arf6-dependent process, and are found in phagocytic vacuoles that interact with endosomes and lysosomes and acquire lysosomal features.[40] During phagocytosis by macrophages, the promastigotes inhibit the formation of the phagolysosome, a cellular product by which invading pathogens are removed. The promastigote can do this using its glycolipid lipophosphoglycan (LPG) on its cell membrane. LPG causes disorganisation of F-actin and disruption of phagosomal lipid microdomains.[41] They are capable of evading the microbicidal actions of macrophages, which can kill ordinary pathogens using reactive nitrogen and oxygen intermediates. They effectively subvert the production of reactive oxygen species. In this way the amastigotes are able to survive and replicate inside these primary immune systems. The parasites manipulate the cell signalling pathway of the macrophages, such as down-regulating of Jak/stat signalling, NO and TNF-α production, and also by blocking the NF-κB-dependent pathway.[42] There are two major mechanisms of immune evasion such as induction of immune suppressive IL-10 responses and the generation of poor and functionally impaired CD8(+) T-cell responses.[43]
Treatment
The conventional treatment method is an intravenous injection with antimony compounds, such as pentostam. Unfortunately, these chemotherapeutics are so poisonous that about 15% of the patients die from the treaments. To compound the situation, drug resistance has evolved in the parasites against the traditional antimonials. According to rough estimates, about 40% of patients in India are already resistant to this therapy.[36] Another antimicrobial drug amphotericin B is also commonly used. Liposomal amphotericin B (L-AmB) has been a drug of choice in India, but is practically useless in Africa because of low effectiveness in the African strain of the parasite.[44] Further, amphotericin B has severe adverse effects. Its acute effects includes nausea, vomiting, rigors, fever, hypertension or hypotension, and hypoxia, and its chronic effect is nephrotoxicity.[45] In 1999 an anticancer drug miltefosine was demonstrated to be highly effective (95% cure rate) among Indian patients.[46] This was the first time an oral drug is effective for visceral leishmaniasis. Clinical trials showed that the new drug is relatively harmless. The most adverse effects were only vomiting and diarrhoea in 20–28% patients, which were rather mild. The drug has been officially approved in India. The recommended dosage is 100 mg per day over a period of four weeks.[47][48]
Evolution
L. donovani is now considered to be a complex species as indicated by different pathological symptoms occurring in different geographical areas where the species of the vector sandfly are also different. However, none of the parasites are morphologically distinguishable, except by molecular analysis. Molecular data show that genotype is strongly correlated with geographical origin.[49] DNA sequencing of different geographical strains indicates that the protozoan complex can be classified into two valid taxons, L. donovani and L. infantum. The genus Leishmania most likely originated in South America, from where it migrated to Asia. L. donovani and L. infantum diverged ~1 Mya, with further divergence of infraspecific genetic groups between 0.4 and 0.8 Mya.[50]
References
- ↑ van Griensven, Johan; Diro, Ermias (June 2012). "Visceral Leishmaniasis". Infectious Disease Clinics of North America. 26 (2): 309–322. doi:10.1016/j.idc.2012.03.005. PMID 22632641.
- ↑ Evans, TG (Sep 1993). "Leishmaniasis.". Infectious Disease Clinics of North America. 7 (3): 527–46. PMID 8254158.
- ↑ Herwaldt, BL (1999). "Leishmaniasis.". Lancet. 354 (9185): 1191–9. doi:10.1016/S0140-6736(98)10178-2. PMID 10513726.
- 1 2 Desjeux, P (2004). "Leishmaniasis: current situation and new perspectives". Comparative Immunology, Microbiology and Infectious Diseases. 27 (5): 305–18. doi:10.1016/j.cimid.2004.03.004. PMID 15225981.
- ↑ Engwerda, CR; Ato, M; Kaye, PM (2004). "Macrophages, pathology and parasite persistence in experimental visceral leishmaniasis". Trends in Parasitology. 20 (11): 524–30. doi:10.1016/j.pt.2004.08.009. PMID 15471704.
- ↑ Lodge, R; Descoteaux, A (2008). "Leishmania invasion and phagosome biogenesis". Sub-cellular biochemistry. 47: 174–81. doi:10.1007/978-0-387-78267-6_14. PMID 18512351.
- ↑ Downing, T; Imamura, H; Decuypere, S; Clark, TG; Coombs, GH; Cotton, JA; Hilley, JD; de Doncker, S; Maes, I; Mottram, JC; Quail, MA; Rijal, S; Sanders, M; Schönian, G; Stark, O; Sundar, S; Vanaerschot, M; Hertz-Fowler, C; Dujardin, JC; Berriman, M (2011). "Whole genome sequencing of multiple Leishmania donovani clinical isolates provides insights into population structure and mechanisms of drug resistance". Genome Research. 21 (12): 2143–56. doi:10.1101/gr.123430.111. PMC 3227103
 . PMID 22038251. Cite uses deprecated parameter
. PMID 22038251. Cite uses deprecated parameter |coauthors=(help) - ↑ "1903-1917". American Society for Microbiology. Retrieved 28 January 2014.
- ↑ S. Tharakaram. "CHARLES DONOVAN, MD, Indian Medical Service" (PDF). www.evolve360.co.uk. Liverpool Medical History Society. Retrieved 28 January 2014.
- ↑ Burma, edited by D.P.; Chakravorty, Maharani (2011). From Physiology and Chemistry to Biochemistry. Delhi: Longman. pp. 36–37. ISBN 9788131732205.
- ↑ Pati, [edited by] Biswamoy; Harrison, Mark (2009). The Social History of Health and Medicine in Colonial India (Reprinted. ed.). London: Routledge. p. 99. ISBN 9780203886984.
- 1 2 3 Richard D. Pearson; Selma M.B. Jeronimo; Anastacio de Q. Sousa (2001). "Leishmaniasis". In Stephen H. Gillespie, Richard D. Principles and Practice of Clinical Parasitology. Chichester: John Wiley & Sons. pp. 287–290. ISBN 9780470851722.
- 1 2 "Morphology and Life Cycle". UCLA. Retrieved 24 January 2014.
- ↑ Pulvertaft, RJ; Hoyle, GF (1960). "Stages in the life-cycle of Leishmania donovani". Transactions of the Royal Society of Tropical Medicine and Hygiene. 54 (2): 191–6. doi:10.1016/0035-9203(60)90057-2. PMID 14435316.
- ↑ Ansari MY, Equbal A, Dikhit MR, Mansuri R, Rana S, Ali V, Sahoo GC, Das P (Nov 2015). "Establishment of Correlation between In-Silico &In-Vitro Test Analysis against Leishmania HGPRT to inhibitors". International Journal of Biological Macromolecules. doi:10.1016/j.ijbiomac.2015.11.051. PMID 26616453.
- ↑ Franke, ED; McGreevy, PB; Katz, SP; Sacks, DL (1985). "Growth cycle-dependent generation of complement-resistant Leishmania promastigotes". Journal of immunology (Baltimore, Md. : 1950). 134 (4): 2713–8. PMID 3973390.
- ↑ Pearson, RD; Romito, R; Symes, PH; Harcus, JL (1981). "Interaction of Leishmania donovani promastigotes with human monocyte-derived macrophages: parasite entry, intracellular survival, and multiplication". Infection and Immunity. 32 (3): 1249–53. PMC 351586
 . PMID 7251165.
. PMID 7251165. - ↑ Chang, KP; Fong, D (1983). "Cell biology of host-parasite membrane interactions in leishmaniasis". Ciba Foundation Symposium. 99: 113–37. doi:10.1002/9780470720806.ch7. PMID 6357669.
- ↑ Pearson, RD; Steigbigel, RT (1981). "Phagocytosis and killing of the protozoan Leishmania donovani by human polymorphonuclear leukocytes". Journal of Immunology (Baltimore, Md. : 1950). 127 (4): 1438–43. PMID 7276565.
- ↑ Howells, RE; Gardener, PJ (1972). "Preliminary electron microscope observations on the amastigote forms of Trypanosoma cruzi and Leishmania donovani". Transactions of the Royal Society of Tropical Medicine and Hygiene. 66 (2): 336. doi:10.1016/0035-9203(72)90214-3. PMID 4558827.
- 1 2 3 Chatterjee, K.D. (2009). Parasitology: Protozoology and Helminthology (13th ed.). New Delhi: CBC Publishers. pp. 67–72. ISBN 9788123918105.
- ↑ Pulvertaft, R.J.V.; Hoyle, G.F. (1960). "Stages in the life-cycle of Leishmania donovani". Transactions of the Royal Society of Tropical Medicine and Hygiene. 54 (2): 191–196. doi:10.1016/0035-9203(60)90057-2. PMID 14435316.
- ↑ Rogers MB, Downing T, Smith BA, Imamura H, Sanders M, Svobodova M, Volf P, Berriman M, Cotton JA, Smith DF (January 2014). "Genomic confirmation of hybridisation and recent inbreeding in a vector-isolated Leishmania population". PLoS Genet. 10 (1): e1004092. doi:10.1371/journal.pgen.1004092. PMC 3894156
 . PMID 24453988.
. PMID 24453988. - ↑ Chappuis, François; Sundar, Shyam; Hailu, Asrat; Ghalib, Hashim; Rijal, Suman; Peeling, Rosanna W.; Alvar, Jorge; Boelaert, Marleen (2007). "Visceral leishmaniasis: what are the needs for diagnosis, treatment and control?". Nature Reviews Microbiology. 5 (11): S7–S16. doi:10.1038/nrmicro1748.
- ↑ Sacks, DL (2001). "Leishmania-sand fly interactions controlling species-specific vector competence". Cellular Microbiology. 3 (4): 189–96. doi:10.1046/j.1462-5822.2001.00115.x. PMID 11298643.
- ↑ Bates, PA; Dwyer, DM (1987). "Biosynthesis and secretion of acid phosphatase by Leishmania donovani promastigotes". Molecular and Biochemical Parasitology. 26 (3): 289–96. doi:10.1016/0166-6851(87)90081-8. PMID 3323906.
- ↑ Ilg, T; Stierhof, YD; Wiese, M; McConville, MJ; Overath, P (1994). "Characterization of phosphoglycan-containing secretory products of Leishmania". Parasitology. 108 (Suppl): S63–71. doi:10.1017/s0031182000075739. PMID 8084657.
- ↑ Hassan, Mo'awia M; Osman, Omran F; El-Raba'a, Fathi MA; Schallig, Henk DFH; Elnaiem, Dia-Eldin A (2009). "Role of the domestic dog as a reservoir host of Leishmania donovani in eastern Sudan". Parasites & Vectors. 2 (1): 26. doi:10.1186/1756-3305-2-26. PMC 2706818
 . PMID 19534802.
. PMID 19534802. - ↑ Ashford, RW (1996). "Leishmaniasis reservoirs and their significance in control". Clinics in Dermatology. 14 (5): 523–32. doi:10.1016/0738-081x(96)00041-7. PMID 8889331.
- ↑ Shaw, JJ (1988). "Animal reservoirs of Leishmania in different ecological situations and their importance in the epidemiology of the disease". Memorias do Instituto Oswaldo Cruz. 83 (Suppl 1): 486–90. doi:10.1590/s0074-02761988000500054. PMID 3253511.
- ↑ Lainson, R; Rangel, EF (2005). "Lutzomyia longipalpis and the eco-epidemiology of American visceral leishmaniasis, with particular reference to Brazil: a review". Memorias do Instituto Oswaldo Cruz. 100 (8): 811–27. doi:10.1590/S0074-02762005000800001. PMID 16444411.
- ↑ Desjeux, P (May–Jun 2001). "The increase in risk factors for leishmaniasis worldwide". Transactions of the Royal Society of Tropical Medicine and Hygiene. 95 (3): 239–43. doi:10.1016/S0035-9203(01)90223-8. PMID 11490989.
- ↑ Blackwell, J. M.; Fakiola, M.; Ibrahim, M. E.; Jamieson, S. E.; Jeronimo, S. B.; Miller, E. N.; Mishra, A.; Mohamed, H. S.; Peacock, C. S.; Raju, M.; Sundar, S.; Wilson, M. E. (May 2009). "Genetics and visceral leishmaniasis: of mice and man". Parasite Immunology. 31 (5): 254–266. doi:10.1111/j.1365-3024.2009.01102.x. PMC 3160815
 . PMID 19388946.
. PMID 19388946. - ↑ Mahajan R.C.; Mohan K. (1996). "Epidemiology of visceral leishmaniasis and its control". In M. Ziya Alkan; M. Ali Özcel. Parasitology for the 21st Century. Wallingford: CAB International. pp. 41–49. ISBN 9780851989778.
- ↑ Seaman, J; Mercer, AJ; Sondorp, E (1996). "The epidemic of visceral leishmaniasis in western Upper Nile, southern Sudan: course and impact from 1984 to 1994". International Journal of Epidemiology. 25 (4): 862–71. doi:10.1093/ije/25.4.862. PMID 8921468.
- 1 2 "Cure for Fatal Tropical Disease - Oral Treatment of Leishmaniasis". Max Planck Institute for Biophysical Chemistry. 18 February 2000. Retrieved 25 January 2014.
- ↑ Stanley, AC; Engwerda, CR (2007). "Balancing immunity and pathology in visceral leishmaniasis". Immunology and Cell Biology. 85 (2): 138–47. doi:10.1038/sj.icb7100011. PMID 17146466.
- ↑ Okwor, I; Uzonna, JE (May 2013). "The immunology of Leishmania/HIV co-infection". Immunologic Research. 56 (1): 163–71. doi:10.1007/s12026-013-8389-8. PMID 23504228.
- ↑ Olivier, M; Badaró, R; Medrano, FJ; Moreno, J (2003). "The pathogenesis of Leishmania/HIV co-infection: cellular and immunological mechanisms". Annals of Tropical Medicine and Parasitology. 97 (Suppl 1): 79–98. doi:10.1179/000349803225002561. PMID 14678636.
- ↑ Lodge, Robert; Descoteaux, Albert (2006). "Phagocytosis of Leishmania donovani amastigotes is Rac1 dependent and occurs in the absence of NADPH oxidase activation". European Journal of Immunology. 36 (10): 2735–2744. doi:10.1002/eji.200636089. PMID 16955522.
- ↑ Moradin, Neda; Descoteaux, Albert (2012). "Leishmania promastigotes: building a safe niche within macrophages". Frontiers in Cellular and Infection Microbiology. 2: 2:121. doi:10.3389/fcimb.2012.00121. PMC 3445913
 . PMID 23050244.
. PMID 23050244. - ↑ Shadab, Md.; Ali, Nahid (2011). "Evasion of host defence by Leishmania donovani: subversion of signaling pathways". Molecular Biology International. 2011: 1–10. doi:10.4061/2011/343961.
- ↑ Stäger, Simona; Joshi, Trupti; Bankoti, Rashmi (2010). "Immune evasive mechanisms contributing to persistent Leishmania donovani infection". Immunologic Research. 47 (1-3): 14–24. doi:10.1007/s12026-009-8135-4. PMID 20087685.
- ↑ Sundar, Shyam; Chakravarty, Jaya (2013). "Leishmaniasis: an update of current pharmacotherapy". Expert Opinion on Pharmacotherapy. 14 (1): 53–63. doi:10.1517/14656566.2013.755515. PMID 23256501.
- ↑ Laniado-Laborín, Rafael; Cabrales-Vargas, Maria Noemí (2009). "Amphotericin B: side effects and toxicity". Revista Iberoamericana de Micología. 26 (4): 223–227. doi:10.1016/j.riam.2009.06.003. PMID 19836985.
- ↑ Jha, TK; Sundar, S; Thakur, CP; Bachmann, P; Karbwang, J; Fischer, C; Voss, A; Berman, J (1999). "Miltefosine, an oral agent, for the treatment of Indian visceral leishmaniasis". The New England Journal of Medicine. 341 (24): 1795–800. doi:10.1056/NEJM199912093412403. PMID 10588964.
- ↑ Sundar, S; Jha, TK; Thakur, CP; Bhattacharya, SK; Rai, M (2006). "Oral miltefosine for the treatment of Indian visceral leishmaniasis". Transactions of the Royal Society of Tropical Medicine and Hygiene. 100 (Suppl 1): S26–33. doi:10.1016/j.trstmh.2006.02.011. PMID 16730038.
- ↑ Sindermann, H; Croft, SL; Engel, KR; Bommer, W; Eibl, HJ; Unger, C; Engel, J (2004). "Miltefosine (Impavido): the first oral treatment against leishmaniasis". Medical Microbiology and Immunology. 193 (4): 173–80. doi:10.1007/s00430-003-0201-2. PMID 14513375.
- ↑ Schönian, G; Kuhls, K; Mauricio, IL (Apr 2011). "Molecular approaches for a better understanding of the epidemiology and population genetics of Leishmania". Parasitology. 138 (4): 405–25. doi:10.1017/S0031182010001538. PMID 21078222.
- ↑ Lukes, J.; Mauricio, I. L.; Schonian, G.; Dujardin, J.-C.; Soteriadou, K.; Dedet, J.-P.; Kuhls, K.; Tintaya, K. W. Q.; Jirku, M.; Chocholova, E.; Haralambous, C.; Pratlong, F.; Obornik, M.; Horak, A.; Ayala, F. J.; Miles, M. A. (2007). "Evolutionary and geographical history of the Leishmania donovani complex with a revision of current taxonomy". Proceedings of the National Academy of Sciences. 104 (22): 9375–9380. doi:10.1073/pnas.0703678104. PMC 1890502
 . PMID 17517634. Cite uses deprecated parameter
. PMID 17517634. Cite uses deprecated parameter |coauthors=(help)
External links
- NCBI taxonomy
- Taxonomy at UniProt
- Genome information in EBI
- Information at Centers for Disease Control and Prevention
- Brief account
- Transmission of visceral leishmaniasis
- Visceral leishmaniasis at Stanford
- details Encyclopedia of Life
- Taxonomy at BioLib
