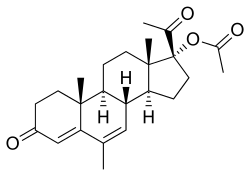
Megestrol acetate
 | |
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral (tablets, suspension) |
| ATC code | G03AC05 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | poor[1][2] |
| Protein binding | Majority to albumin (no affinity for SHBG or CBG)[1][2] |
| Biological half-life | 13–105 hours (mean 34)[3] |
| Identifiers | |
| |
| Synonyms | BDH-1298, NSC-71423[4] |
| CAS Number | 595-33-5 |
| PubChem (CID) | 11683 |
| DrugBank | DB00351 |
| ChemSpider | 11192 |
| Chemical and physical data | |
| Formula | C24H32O4 |
| Molar mass | 384.509 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
Megestrol acetate (MGA) (INN, USAN, BAN, JAN) (brand names Megace, Megace ES), also known as 17α-acetoxy-6-dehydro-6-methylprogesterone, is a steroidal progestin of the 17α-hydroxyprogesterone group that is used in the treatment of breast and endometrial cancer and as an appetite stimulant.[5][6][7] It is the 17α-acetate ester of megestrol, which, in contrast to MGA, was never marketed for clinical use.[5][6] The term megestrol is often inappropriately used as a synonym for MGA, and when it is used, it almost always refers to MGA rather actually than megestrol.
Medical uses
MGA is used mainly as an appetite stimulant in a variety of conditions and as an antineoplastic agent in the treatment of breast, endometrial, and prostate cancers.[8] When given in relatively high doses, it can substantially increase appetite in most individuals, even those with advanced cancer, and is often used to boost appetite and induce weight gain in patients with cancer or HIV/AIDS-associated cachexia. It is also used as a contraceptive in combination with an estrogen at relatively low doses.
In addition to its use in humans, MGA has been used extensively in veterinary medicine in the treatment of medical conditions in cats and dogs.[6]
Available forms
MGA is available as 5 mg, 20 mg and 40 mg tablets and in oral suspensions of 125 mg/ml and 40 mg/ml. It is used at a dose of 5 mg in combination with an estrogen for contraception. Appetite stimulation is achieved with doses ranging from 400 mg to 800 mg/day. Doses used to treat cancer usually range from 40 mg to 320 mg.
Contraindications
MGA should not be used in pregnancy under any circumstance as it crosses the placenta and malignantly affects the fetus.[9]
Side effects
The most common side effect of MGA is weight gain. Other side effects may include nausea, vomiting, nightmares, impotence, edema, breakthrough bleeding, and shortness of breath. Rare and more severe side effects may include thrombophlebitis and pulmonary embolism.[3] It may also cause glucocorticoid-related adverse effects such as adrenal insufficiency in some individuals and/or cases (especially if the medication is suddenly discontinued following prolonged use).[10][11]
Pharmacology
Progestogenic activity
MGA acts predominantly as a potent agonist of the progesterone receptor (PR) to exert its effects.[12]
Antigonadotropic activity
MGA has strong antigonadotropic effects in humans at sufficient doses, capable of dramatically suppressing circulating androgen and estrogen concentrations in both sexes.[13][14][15][16][17] It can also decrease sex hormone receptors in certain parts of the body; as an example, one study in men with benign prostatic hyperplasia who were treated with 120–160 mg MGA per day for 3 to 11 days found average decreases in AR quantity of 73% and 86% in the cytoplasm and nucleus of prostatic cells, respectively.[16] The antigonadotropic effects of MGA are the result of strong activation of the PR, which suppresses the secretion of the gonadotropins—peptide hormones responsible for signaling the body to produce not only progesterone but also the androgens and the estrogens—from the pituitary gland as a form of negative feedback inhibition, and hence downregulates the hypothalamic-pituitary-gonadal (HPG) axis, resulting in decreased levels of the sex hormones.[18] It is the antiandrogenic and antiestrogenic effects of MGA mediated by suppression of the HPG axis that are mainly responsible for its beneficial effects against androgen and estrogen-sensitive cancers, respectively.[19][20]
Antiandrogenic activity
MGA is a high-affinity antagonist/weak partial agonist of the AR,[21][22][23] where it binds with very similar but slightly less affinity relative to the PR (about 75% of the affinity according to one assay).[12] Despite its weak intrinsic activity at the AR, at clinical doses in humans, MGA appears to behave, for all intents and purposes, purely as an antiandrogen. No androgenic side effects have been observed with the use of MGA in patients of either sex at dosages up to as high as 1,600 mg per day (which is the highest that has been used).[24] Furthermore, it produces detectable androgenic effects in animals only at a dose that is the equivalent of approximately 200 times that typically used for the treatment of prostate cancer in men.[25]
Estrogenic activity
Unlike the case of the AR, MGA has no significant affinity for the ER.[12] As such, it does not possess the capacity to directly activate the ER. Furthermore, unlike antiandrogens such as cyproterone acetate and flutamide, there is relatively little risk of indirectly mediated estrogenic side effects (e.g., gynecomastia) with MGA.[26] This is because MGA strongly suppresses both androgen and estrogen levels at the same time.
Glucocorticoid activity
MGA is an agonist of the glucocorticoid receptor (GR), with similar but less affinity in comparison to the PR and the AR (about 37% and 50% of the affinity, respectively, according to one assay).[12][23] One study found that, in the dose range tested, it possesses about 50% of the eosinopenic and hyperglycemic activity (markers of glucocorticoid activity) of an equal amount of medroxyprogesterone acetate, and about 25% that of cortisol.[27] Accordingly, manifestations of its glucocorticoid properties, including symptoms of Cushing's syndrome, steroid diabetes, and adrenal insufficiency, have been reported with the use of MGA in the medical literature, albeit sporadically.[28]
Appetite stimulant
MGA is frequently used as an appetite stimulant. The direct mechanism of appetite enhancement is unclear, but it is known that MGA induces a variety of downstream changes to cause the effect, including stimulation of the release of neuropeptide Y in the hypothalamus, modulation of calcium channels in the ventromedial hypothalamus, and inhibition of the secretion of proinflammatory cytokines including IL-1α, IL-1β, IL-6, and TNF-α, all of which have been implicated in facilitation of appetite.[29][30][31]
History
MGA was first synthesized, in 1959, from medroxyprogesterone acetate, which itself had been synthesized the year prior in 1958.[32] MGA in combination with ethinyl estradiol (EE) was introduced in 1963 by British Drug Houses in the United Kingdom under the brand name Volidan (4 mg MGA and 50 μg EE tablets) as an oral contraceptive,[33][34] and this was followed by Serial 28 (1 mg MGA and 100 μg EE tablets) and Volidan 21 (4 mg MGA and 50 μg EE tablets) in 1964 and Nuvacon (2 mg MGA and 100 μg EE tablets) in 1967, all by British Drug Houses also in the U.K.[35] MGA was approved in 1967 for the treatment of breast cancer.[36][37] In the 1970s, it was found to be associated with mammary tumors in beagle dogs, and along with several other progestogens, was withdrawn from several markets as an oral contraceptive.[36] Subsequent research revealed that there is no similar risk in humans.[38]
See also
- Anagestone acetate
- Chlormadinone acetate
- Cyproterone acetate
- Delmadinone acetate
- Melengestrol acetate
- Nomegestrol acetate
References
- 1 2 Schindler AE, Campagnoli C, Druckmann R, et al. (December 2003). "Classification and pharmacology of progestins". Maturitas. 46 Suppl 1: S7–S16. doi:10.1016/j.maturitas.2003.09.014. PMID 14670641.
- 1 2 Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- 1 2 Richard R. Barakat; Maurie Markman; Marcus Randall (29 May 2009). Principles and Practice of Gynecologic Oncology. Lippincott Williams & Wilkins. p. 447. ISBN 978-0-7817-7845-9. Retrieved 2 June 2012.
- ↑ Dr. Ian Morton; Ian K. M. Morton; Judith M. Hall; Dr. Judith Hall (1999). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer. p. 173. ISBN 978-0-7514-0499-9. Retrieved 2 June 2012. Cite uses deprecated parameter
|coauthors=(help) - 1 2 F.. Macdonald (1997). Dictionary of Pharmacological Agents. CRC Press. p. 1267. ISBN 978-0-412-46630-4. Retrieved 12 May 2012.
- 1 2 3 Index Nominum 2000: International Drug Directory. Taylor & Francis US. 2000. p. 641. ISBN 978-3-88763-075-1. Retrieved 2 June 2012.
- ↑ Neumann F (1978). "The physiological action of progesterone and the pharmacological effects of progestogens--a short review". Postgraduate Medical Journal. 54 Suppl 2: 11–24. PMID 368741.
- ↑ Kenneth L. Becker (24 April 2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. p. 1195. ISBN 978-0-7817-1750-2. Retrieved 27 May 2012.
- ↑ http://www.answers.com/topic/megestrol-megace
- ↑ Chidakel AR, Zweig SB, Schlosser JR, Homel P, Schappert JW, Fleckman AM (February 2006). "High prevalence of adrenal suppression during acute illness in hospitalized patients receiving megestrol acetate". Journal of Endocrinological Investigation. 29 (2): 136–40. doi:10.1007/bf03344086. PMID 16610239.
- ↑ Bulchandani D, Nachnani J, Amin A, May J (August 2008). "Megestrol acetate-associated adrenal insufficiency". The American Journal of Geriatric Pharmacotherapy. 6 (3): 167–72. doi:10.1016/j.amjopharm.2008.08.004. PMID 18775392.
- 1 2 3 4 Teulings FA, van Gilse HA, Henkelman MS, Portengen H, Alexieva-Figusch J (July 1980). "Estrogen, androgen, glucocorticoid, and progesterone receptors in progestin-induced regression of human breast cancer". Cancer Research. 40 (7): 2557–61. PMID 6248208.
- ↑ Geller J, Albert J, Yen SS, Geller S, Loza D (March 1981). "Medical castration of males with megestrol acetate and small doses of diethylstilbestrol". The Journal of Clinical Endocrinology and Metabolism. 52 (3): 576–80. doi:10.1210/jcem-52-3-576. PMID 6161942.
- ↑ Venner PM (December 1990). "Therapeutic options in treatment of advanced carcinoma of the prostate". Seminars in Oncology. 17 (6 Suppl 9): 73–7. PMID 2259929.
- ↑ Lundgren S, Lønning PE, Utaaker E, Aakvaag A, Kvinnsland S (June 1990). "Influence of progestins on serum hormone levels in postmenopausal women with advanced breast cancer--I. General findings". Journal of Steroid Biochemistry. 36 (1-2): 99–104. doi:10.1016/0022-4731(90)90118-c. PMID 2362454.
- 1 2 Geller J, Albert J, Geller S (1982). "Acute therapy with megestrol acetate decreases nuclear and cytosol androgen receptors in human BPH tissue". The Prostate. 3 (1): 11–5. doi:10.1002/pros.2990030103. PMID 6176985.
- ↑ Blumenschein GR (December 1983). "The role of progestins in the treatment of breast cancer". Seminars in Oncology. 10 (4 Suppl 4): 7–10. PMID 6230722.
- ↑ Alexieva-Figusch J, Blankenstein MA, de Jong FH, Lamberts SW (September 1984). "Endocrine effects of the combination of megestrol acetate and tamoxifen in the treatment of metastatic breast cancer". European Journal of Cancer & Clinical Oncology. 20 (9): 135–40. PMID 6434315.
- ↑ Schacter L, Rozencweig M, Canetta R, Kelley S, Nicaise C, Smaldone L (March 1989). "Megestrol acetate: clinical experience". Cancer Treatment Reviews. 16 (1): 49–63. doi:10.1016/0305-7372(89)90004-2. PMID 2471590.
- ↑ Sedlacek SM (April 1988). "An overview of megestrol acetate for the treatment of advanced breast cancer". Seminars in Oncology. 15 (2 Suppl 1): 3–13. PMID 3285483.
- ↑ Eil C, Edelson SK (July 1984). "The use of human skin fibroblasts to obtain potency estimates of drug binding to androgen receptors". The Journal of Clinical Endocrinology and Metabolism. 59 (1): 51–5. doi:10.1210/jcem-59-1-51. PMID 6725525.
- ↑ Luthy IA, Begin DJ, Labrie F (November 1988). "Androgenic activity of synthetic progestins and spironolactone in androgen-sensitive mouse mammary carcinoma (Shionogi) cells in culture". Journal of Steroid Biochemistry. 31 (5): 845–52. doi:10.1016/0022-4731(88)90295-6. PMID 2462135.
- 1 2 Poyet P, Labrie F (October 1985). "Comparison of the antiandrogenic/androgenic activities of flutamide, cyproterone acetate and megestrol acetate". Molecular and Cellular Endocrinology. 42 (3): 283–8. doi:10.1016/0303-7207(85)90059-0. PMID 3930312.
- ↑ Farrar DJ (March 1999). "Megestrol acetate: promises and pitfalls". AIDS Patient Care and STDs. 13 (3): 149–52. doi:10.1089/apc.1999.13.149. PMID 10375262.
- ↑ Tisell LE, Salander H (February 1975). "Androgenic properties and adrenal depressant activity of megestrol acetate observed in castrated male rats". Acta Endocrinologica. 78 (2): 316–24. doi:10.1530/acta.0.0780316. PMID 1172901.
- ↑ Kenneth A. Foon (1998). Biological and Hormonal Therapies of Cancer. Springer. p. 73. ISBN 978-0-7923-9997-1. Retrieved 2 June 2012.
- ↑ Briggs MH, Briggs M (October 1973). "Glucocorticoid properties of progestogens". Steroids. 22 (4): 555–9. doi:10.1016/0039-128x(73)90011-1. PMID 4747450.
- ↑ Mann M, Koller E, Murgo A, Malozowski S, Bacsanyi J, Leinung M (1997). "Glucocorticoidlike activity of megestrol. A summary of Food and Drug Administration experience and a review of the literature". Archives of Internal Medicine. 157 (15): 1651–6. doi:10.1001/archinte.157.15.1651. PMID 9250225.
- ↑ Ann M. Berger; John L. Shuster; Jamie H. Von Roenn (6 October 2006). Principles And Practice of Palliative Care And Supportive Oncology. Lippincott Williams & Wilkins. p. 128. ISBN 978-0-7817-9595-1. Retrieved 27 May 2012.
- ↑ Achim Jörres (19 February 2010). Management of Acute Kidney Problems. Springer. p. 210. ISBN 978-3-540-69413-7. Retrieved 27 May 2012.
- ↑ David S. Ettinger (11 November 2008). Supportive Care in Cancer Therapy. Springer. p. 61. ISBN 978-1-58829-941-3. Retrieved 27 May 2012.
- ↑ Benign Prostatic Hypertrophy. Springer Science & Business Media. 6 December 2012. pp. 277–. ISBN 978-1-4612-5476-8.
- ↑ Lara Marks (2010). Sexual Chemistry: A History of the Contraceptive Pill. Yale University Press. pp. 77–78. ISBN 978-0-300-16791-7.
- ↑ MEARS E (1963). "A new type of oral contraceptive". Br Med J. 1 (5341): 1318–20. PMC 2123904
 . PMID 13934321.
. PMID 13934321. - ↑ Lara Marks (2001). Sexual Chemistry: A History of the Contraceptive Pill. Yale University Press. pp. 78–. ISBN 978-0-300-08943-1.
- 1 2 Consolidated List of Products Whose Consumption And/or Sale Have Been Banned, Withdrawn, Severely Restricted Or Not Approved by Governments. United Nations Publications. 1983. pp. 137–. ISBN 978-92-1-130230-1.
- ↑ Hong, Soon Wook; Lee, Bong Sang; Park, Su Jun; Jeon, Hong Ryeol; Moon, Ki Young; Kang, Mean Hyung; Park, Sang Han; Choi, Sung-Up; Song, Woo Heon; Lee, Jaehwi; Choi, Young Wook (2011). "Solid dispersion formulations of megestrol acetate with copovidone for enhanced dissolution and oral bioavailability". Archives of Pharmacal Research. 34 (1): 127–135. doi:10.1007/s12272-011-0115-2. ISSN 0253-6269.
- ↑ Benno Clemens Runnebaum; Thomas Rabe; Ludwig Kiesel (6 December 2012). Female Contraception: Update and Trends. Springer Science & Business Media. pp. 134–. ISBN 978-3-642-73790-9.