Tiletamine
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | IV, IM, SC, Other |
| ATC code | none |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Liver |
| Excretion | Kidneys |
| Identifiers | |
| |
| CAS Number |
14176-50-2 |
| PubChem (CID) | 26533 |
| ChemSpider |
24714 |
| UNII |
2YFC543249 |
| KEGG |
D08596 |
| ECHA InfoCard | 100.034.559 |
| Chemical and physical data | |
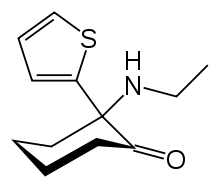
| Formula | C12H17NOS |
| Molar mass | 223.34 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Tiletamine is a dissociative anesthetic and pharmacologically classified as an NMDA receptor antagonist.[1] It is related chemically to ketamine.[2] Tiletamine hydrochloride exists as odourless white crystals.
It is used in veterinary medicine in the compound product Telazol (tiletamine/zolazepam, 50 mg/ml of each in 5 ml vial) as an injectable anesthetic for use in cats and dogs.[3][4][5] It is sometimes used in combination with xylazine (Rompun) to tranquilize large mammals such as polar bears[6] and wood bison.[7] Telazol is the only commercially available tiletamine product in the United States. It is contraindicated in patients of an ASA score of III or greater and in animals with CNS signs, hyperthyroidism, cardiac disease, pancreatic or renal disease, pregnancy, glaucoma, or penetrating eye injuries.[3]
Abuse of Telazol has been documented.[8] Animal studies have also shown that tiletamine produces rewarding and reinforcing effects.[9] Tiletamine products are classified as Schedule III controlled substances in the United States.[10]
See also
-
Pharmacy and Pharmacology portal
- Arylcyclohexylamine
- Dissociatives
- NMDA receptor antagonist
- Ketamine
References
- ↑ Klockgether, Thomas; Turski, Lechoslaw; Schwarz, Michael; Sontag, Karl-Heinz; Lehmann, John (1 October 1988). "Paradoxical convulsant action of a novel non-competitiveN-methyl-d-aspartate (NMDA) antagonist, tiletamine". Brain Research. 461 (2): 343–348. doi:10.1016/0006-8993(88)90265-X. PMID 2846121.
- ↑ NIH Pubchem entry for Tiletamine
- 1 2 "Tiletamine entry at Drugs.com". Retrieved 5 January 2012.
- ↑ Lin HC, Thurmon JC, Benson GJ, Tranquilli WJ. Telazol: a review of its pharmacology and use in veterinary medicine. J Vet Pharmacol Ther. 1993 Dec;16(4):383-418. Review. PMID 8126757
- ↑ NIH Tiletamine entry in Toxnet Page last reviewed 1/21/2009
- ↑ Cattet, M.R.; Caulkett, N.A.; Lunn, N.J. (July 2003). "Anesthesia of polar bears using xylazine-zolazepam-tiletamine or zolazepam-tiletamine.". Journal of wildlife diseases. 39 (3): 655–64. doi:10.7589/0090-3558-39.3.655. PMID 14567228.
- ↑ Caulkett, N.A.; Cattet, M.R.; Cantwell, S.; Cool, N.; Olsen, W. (January 2000). "Anesthesia of wood bison with medetomidine-zolazepam/tiletamine and xylazine-zolazepam/tiletamine combinations.". The Canadian Veterinary Journal/La revue veterinaire canadienne. 41 (1): 49–53. PMC 1476335
 . PMID 10642872.
. PMID 10642872. - ↑ Quail, MT; Weimersheimer, P; Woolf, AD; Magnani, B (2001). "Abuse of telazol: an animal tranquilizer.". Journal of Toxicology: Clinical Toxicology. 39 (4): 399–402. doi:10.1081/clt-100105161. PMID 11527235.
- ↑ de la Peña JB, Lee HC, de la Peña IC, Woo TS, Yoon SY, Lee HL, Han JS, Lee JI, Cho YJ, Shin CY, Cheong JH (2012). "Rewarding and reinforcing effects of the NMDA receptor antagonist-benzodiazepine combination, Zoletil®: difference between acute and repeated exposure". Behavioural Brain Research. 233 (2): 434–42. doi:10.1016/j.bbr.2012.05.038. PMID 22659394.
- ↑ "Lists of: Scheduling Actions, Controlled Substances, Regulated Chemicals" (PDF). Drug Enforcement Administration. Retrieved 5 January 2012.